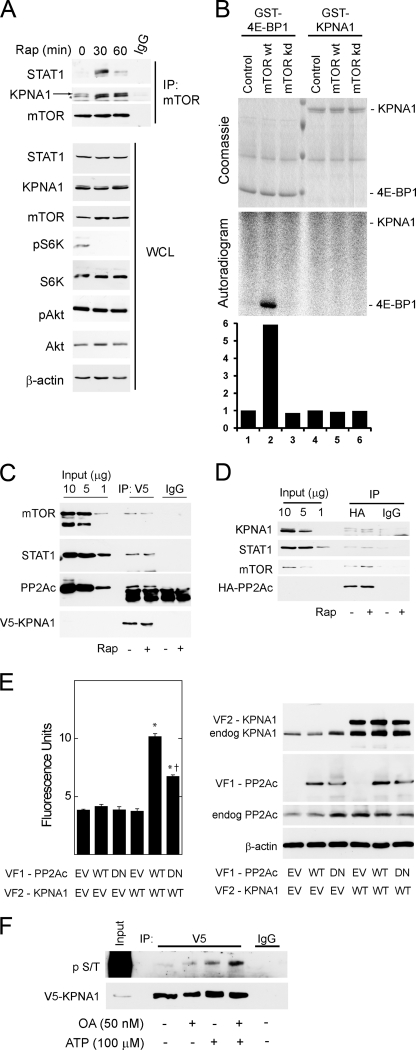
FIGURE 2.
Physical associations between mTOR, its associated phosphatase PP2Ac, and KPNA1 in intact cells. A, HEK 293T cells were exposed to 50 ng/ml rapamycin (Rap) for 0, 30, or 60 min before preparation of whole cell lysates and immunoprecipitation with α-mTOR antibody (IP: mTOR) or normal IgG. Immunoprecipitated proteins or those in whole cell lysates (WCL) were detected by Western blot analysis. The arrow indicates KPNA1, and the asterisk indicates a protein other than KPNA1 that is detected by the anti-KPNA1 antibody. B, HEK 293T cells were transfected with Myc-tagged raptor and either a control vector, wild-type (wt), or kinase inactive (kd) mTOR. After lysis, proteins were immunoprecipitated with α-Myc antibody. mTORC1-containing immunoprecipitates were washed and incubated with [γ-32P]ATP and either recombinant GST-tagged eIF4E-binding protein 1 or KPNA1 in an in vitro kinase reaction. The products were separated by SDS-PAGE before staining with Coomassie Blue or autoradiography. The data in the lower panel show the fold changes in band density (lanes 1–6) versus control = 1 (lane 1). C, HEK 293T cells expressing V5-ECFP-KPNA1 were incubated without or with 50 ng/ml rapamycin (Rap) for 30 min before preparation of whole cell lysates. After immunoprecipitation (IP) with α-V5 antibody, the indicated KPNA1-interacting proteins were detected by Western blot. D, HEK 293T cells expressing HA-PP2Ac were incubated without or with 50 ng/ml rapamycin for 30 min before preparation of whole cell lysates. After immunoprecipitation with anti-HA antibody, the indicated PP2Ac-interacting proteins were detected by Western blot. E, COS7 cells were co-transfected with plasmids for heterologous expression of venus yellow fluorescence protein fragments VF1 or VF2 (empty vector, EV) or fragments linked to wild-type (WT) or dominant negative (DN) PP2Ac or wild-type KPNA1. Direct protein-protein interactions were signaled by increased fluorescence detection upon reconstitution of full-length YFP (excitation, 485 nm; emission, 535 nm; cutoff, 530 nm). Summarized data are the mean fluorescence units/cell ± S.E. in triplicate samples and representative of three individual experiments. *, p < 0.05 versus empty vector control (lane 1); †, p < 0.05 versus lane 5 by Student's t test. Levels of endogenous or recombinant PP2Ac or KPNA1 were detected by Western blot analysis (right panel). F, V5-CFP-KPNA1 heterologously expressed in HEK 293T cells was immunoprecipitated as in C before incubation without or with 100 μm ATP, 50 nm okadaic acid (OA), or both for 1 h at 30 °C. V5 or normal IgG (control) immunoprecipitates were separated by SDS-PAGE before detection of V5-CFP-KPNA1 or phosphoserine/phosphothreonine (p S/T) by Western blot analysis. The images are representative of three individual experiments.