FIGURE 5.
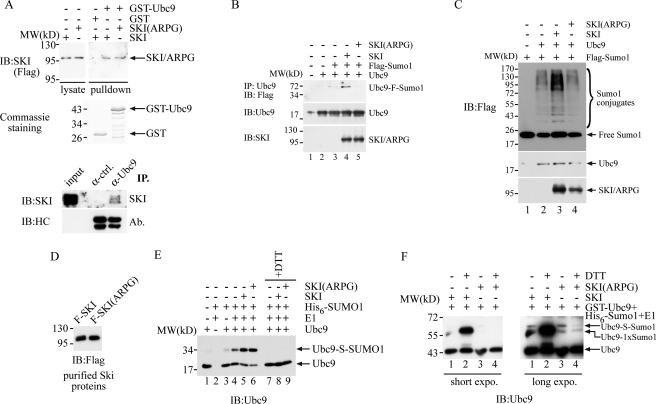
SKI positively regulates Ubc9 both in vivo and in vitro. A, SKI can directly interact with Ubc9. Approximately equal amounts of GST or GST-Ubc9 protein was mixed with lysate prepared from 293T cells with expression of FLAG-SKI, FLAG-SKI(ARPG), or control transfection. The GST fusion protein was retrieved with glutathione-Sepharose beads and washed three times with lysis buffer. The bead-bound proteins were analyzed by immunoblot (IB) analysis with anti-FLAG antibody to reveal the presence of SKI/ARPG protein in the GST-Ubc9 pulldown. The exposure time for the lysate protein was about 1/10 of that for the pulldown. In the lower panel, LNCaP cells were treated with paraformaldehyde for 10 min before collection, and lysed and immunoprecipitated (IP) with anti-Ubc9 antibody or control antibody. The immunoprecipitates were analyzed together with 10 μg of input lysate with SDS-PAGE followed by immunoblot with anti-SKI antibody or antibody for heavy chain. B, SKI enhances Ubc9 sumoylation in vivo. 293T cells were transfected with various plasmids, and the Ubc9 protein was immunoprecipitated with anti-Ubc9 antibody followed by immunoblot with anti-FLAG antibody to reveal the extent of the Ubc9 sumoylation. C, SKI enhances Ubc9-mediated sumoylation in vivo. 293T cells were transfected with various combinations of expression plasmids, and in vivo sumoylation was determined by immunoblot analysis with anti-FLAG antibody using the total lysate after SDS-PAGE. D, purified SKI and SKI(ARPG) protein. SKI and SKI(ARPG) were first immunoprecipitated with anti-FLAG antibody from 293T cells expressing FLAG-Ski or FLAG-Ski(ARPG), and then the immunoprecipitates were washed and incubated with FLAG peptide. The proteins were then quantitated by immunoblot with anti-FLAG antibody. E, SKI increases thioester bond formation of Ubc9 in vitro. 30 ng of Ubc9 protein was mixed with 60 ng of E1, 250 ng of His6-Sumo1, and purified SKI/ARPG protein in 15 μl at 37 °C for 30 min. The reaction mixture was then separated by SDS-PAGE in the presence or absence of DTT in the sample buffer followed by immunoblot analysis with anti-Ubc9 antibody. F, SKI increases mono-sumoylation of Ubc9 in vitro. 10 ng of GST-Ubc9 protein was mixed with 110 ng of E1, 600 ng of His6-Sumo1 as in E followed by immunoblot analysis with anti-Ubc9 antibody. The designation of Ubc9 conjugated with one SUMO1 molecule is based on its molecular weight increase and resistance to DTT.