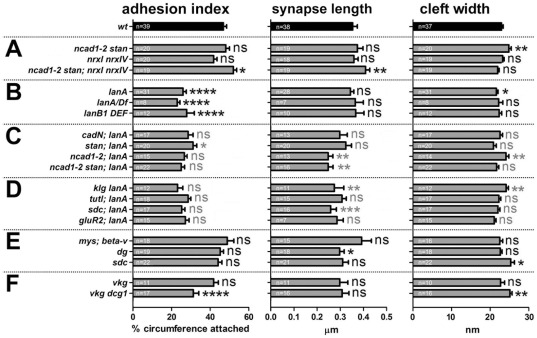
Figure 2. Quantifications of ultrastructural NMJ phenotypes.
Embryonic NMJ boutons displaying active zones (arrows in Figs. 1, 4 and 5) were measured. Genotypes are grouped into combinations of cadherins and neurexins (A), Laminin-deficient conditions (B), combinations of lanA9.32 with loss of cadherins (C), loss of other potential adhesion factors (as explained below) in combination with lanA9.32 (D), loss of classical laminin receptors (E), and collagen type IV-deficient conditions (F). The following parameters were analysed: “adhesion index”, the percentage of the circumference of active zone-bearing boutons that is in contact with muscle membrane (between curved arrows in Figs. 1, 4 and 5); “synapse length”, mean length of electron dense cleft material known to indicate synapse diameter (between double chevrons in Figs. 1, 4 and 5); “cleft width”, mean distance between pre- and postsynaptic membranes at synapses. Bars represent mean ± standard error of the mean; n, number of assessed NMJ boutons sampled from at least 5 embryos, respectively; asterisks indicate statistical significances as compared to wt (black asterisks) or lanA (grey asterisks; *, P≤0.1; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001 according to Mann Whitney tests). Additional information on included CAMs not explained in the main text: the immunoglobulin adhesion receptor Klingon is suggested to express potential synaptic functions [88], [89]; the immunoglobulin adhesion receptor Turtle acts as a homophilic adhesion factor in S2 cell assays which has demonstrated neuronal phenotypes in vivo [91], [92], [104], [105]; the transmembrane heparan sulfate proteoglycan Syndecan (Sdc) might act as a CAM by serving as a ligand for the motorneuronal receptor Lar (Leukocyte-antigen-related-like, a close homolog of avian protein tyrosin phosphatase ó) [106]–[108].