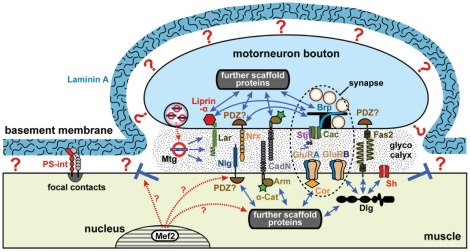
Figure 6. A model view of the embryonic Drosophila NMJ.
In late Drosophila embryos, presynaptic motorneuronal boutons (blue) are attached with half of their surfaces to muscles (beige), and synapses (dashed ellipse) are assembled at these neuromuscular cell-cell contacts. Neuromuscular synapses contain presynaptic active zones with key components such as the scaffolding protein Bruchpilot (Brp) or the Cacophony (Cac) calcium channel including its associated subunit Straightjacket (Stj) [25]. Postsynaptically, neuromuscular synapses contain clusters of GluRs composed of the three obligatory C, D and E subunits and the variable A and B subunits. For most CAMs, such as Leukocyte-antigen-related-like (Lar) [107], Neuroligins (Nlg) [110], [111], Neurexins (Nrx; as mentioned in text), classical cadherins (CadN; as mentioned in text), it remains to be clarified whether they localise within synapses or extra-synaptically; for Fasciclin2 (Fas2) peri-synaptic localisation has already been reported [112]. All these components are interlinked through intracellular scaffolds. Discs large (Dlg) selectively stabilises GluRB receptors at the synapse, but also anchors Shaker potassium channels (Sh) or Fas2 [26], [113]. The band 4.1 superfamily protein Coracle (Cora) interacts with the carboxy-terminus of GluRIIA but not GluRIIB [114], but has likewise been shown to interact with Nrx-IV in other cellular contexts [115]. Links of the Lar-associated scaffold protein Liprin-α to Brp, or of Nrx-IV to Brp have been explained elsewhere [25]. Many more interactions with further scaffold proteins on both sides of the junction are to be expected. The glycocalyx (stippled area) within the synaptic cleft forms a third scaffold established through the linkage of carbohydrate-side chains, often mediated through lectins, such as Mind-the-gap (Mtg). BM links in a Laminin A-dependent manner to cell surfaces through yet unidentified receptors (?), although PS-integrin-mediated Laminin A-independent adhesion at focal contacts has been described [28]. BM is likely to compete with motorneuronal terminals for muscle surface, and BM adhesion needs to be excluded from neuromuscular adhesions (blue T) [116]. Proteins downstream of the Mef2 transcription factor are likely to contribute to this process, as is suggested by complete loss of NMJ adhesion in mef2 mutant embryos [70].