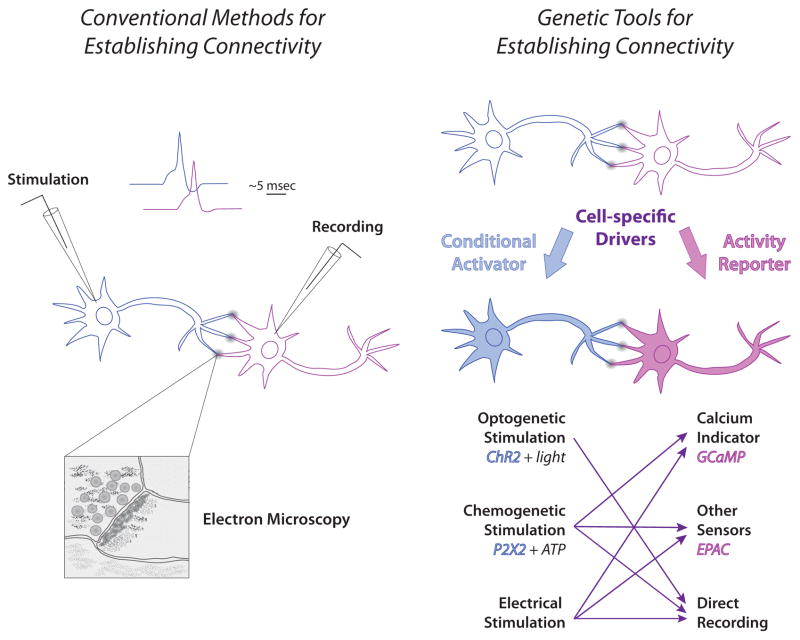
Figure 2.
Methods for establishing functional connectivity. Traditional techniques for establishing connectivity are shown at left. Recording from a putative downstream neuron while electrically stimulating a presynaptic partner allows the investigator to determine if the two neurons are functionally connected, and via analysis of response latency, whether that connection is likely to be direct. In the example shown, the response in the downstream neuron occurs within 5 msec, which is typical for a monosynaptic connection (although temperature and other conditions can affect exact timing). Anatomical verification of a direct connection is accomplished using electron microscopy. Shown on the right are new methods for establishing functional connectivity. In Drosophila, direct recording can be done in some cases, but cell-specific expression systems can be used more generally to express transgenes which activate cells or report activation [9]. There are several methods for conditional activation of putative presynaptic neurons, including channel rhodopsin and the purine receptor P2X2. Responses in downstream cells after electrical or chemical stimulation can be monitored using a variety of genetically encoded fluorescent calcium sensors or reporters that respond to other consequences of presynaptic activity such as EPAC, which senses cyclic nucleotides. The time resolution of the current generation of reporters does not allow unambiguous determination of direct connectivity, and monosynaptic connection still needs to be verified anatomically with either electron microscopy or genetic techniques such as GRASP [39] to demonstrate cell-cell contact. Arrows indicate methods that can be used together. Optogenetic stimulation is not usually compatible with the current fluorescent reporters due to spectral overlap, but new variants are likely to address this incompatibility.