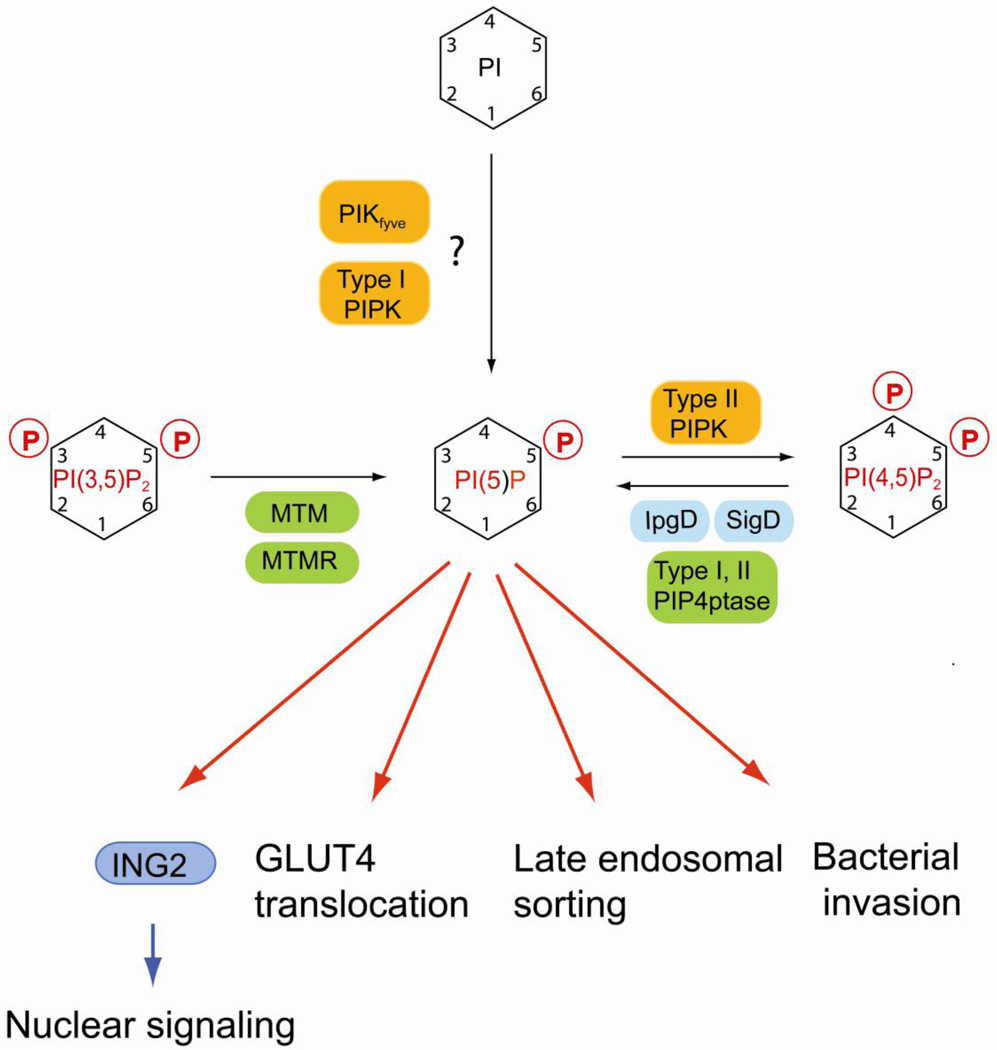
Fig. 5.
Regulation of PI(5)P and its role in nuclear signaling and membrane dynamics. PI(5)P can be synthesized by MTM and MTMR phosphatases via dephosphorylation of PI(3,5)P2 Salmonella and Shigella bacterial pathogens express well characterized lipid phosphatases (IpgD and SigD) that convert PI(4,5)P2 to PI(5)P upon injection into the host cell which is important for the invasion mechanism. Recently, mammalian type I and II PIP 4-phosphatases have been identified that generate PI(5)P in vitro using PI(4,5)P2 as substrate. Biochemical assay suggest that PIKfyve and type I PIP kinase can directly phosphorylate PI to create PI(5)P, but it is unclear whether this reaction is relevant for the physiological regulation of PI(5)P. PI(5)P binds to the nuclear adaptor ING2 that regulates chromatin rearrangement in response to stress. But there is mounting evidence that PI(5)P may play an important role in regulating membrane dynamics in both endocytic and exocytic sorting. Mammalian lipid kinases are marked by orange boxes, lipid phosphatases are in green boxes. Bacterial lipid phosphatases are in cyan boxes. Effector proteins are in blue boxes.