Abstract
AIM
To study the effects of Tetramethylpyrazine (TMP) on retinal pigment epithelium (RPE) degeneration, choroidal blood flow and oxidative stress of RPE cells.
METHODS
The 35mg/kg NaIO3-induced RPE degeneration rat eyes was given 25µg 1% TMP eye drops 3 times a day for 7 days before NaIO3 injection, and then 2 to 4 weeks after NaIO3 injection. RPE function was measured with c-wave of electroretinogram (ERG). Colored microsphere technique was used for in vivo experiments to determine the choroidal blood flow in ocular hypertensive (40mmHg) rabbit eyes. Methylthiazoltetrazolium (MTT) assay was used to study in vitro effect of TMP on various oxidants induced injury in the hRPE (ARPE-19 (ATCC, Manassas, VA, USA)).
RESULTS
Two weeks after NaIO3 injection, the amplitude of ERG c-wave fell markedly in NaIO3 group to 36% of control group(P<0.01). No apparent difference was observed in TMP+NaIO3 group. Four weeks later, the NaIO3 group fell to 46% of control group (P<0.01), while the TMP+NaIO3 group fell to only 77% of control group (P<0.01). There was a 67% reversal of the ERG c-wave by TMP as compared to NaIO3 group(P<0.01). The choroidal blood flow was significantly increased at all time points (at 30, 60 and 120 minutes after TMP instillation) as compared with corresponding controls. TMP had no effect on hypoxia-(1% O2), t-BHP- and H2O2-induced damage in RPE cells. 10(g/mL TMP could reverse 1 and 3mM NaN3-induced loss of viability of RPE by 18.5% (P<0.01) and 23% (P<0.01), respectively. 30µg/mL TMP could reverse 30 and 100mM NaIO3 induced loss of viability of RPE by 18.1% (P<0.05) and 16.8% (P<0.01), respectively.
CONCLUSION
TMP can significantly protect RPE from NaIO3 induced degeneration in vivo and oxidative stress in vitro and can increase choroidal blood flow markedly in vivo.
Keywords: Tetramethylpyrazine, sodium iodate, retinal pigment epithelium, age-related macular degeneration, choroidal blood flow, oxidative stress
INTRODUCTION
A 2004 analysis reported that among Americans over the age of 40, AMD and/or geographic atrophy were present in at least one eye in 1.47% of the population, and that 1.75 million individuals have AMD. The prevalence of AMD increased dramatically with age, with more than 15% of the white women older than 80 years having neovascular AMD and/or geographic atrophy. More than 7 million individuals are at substantial risk of developing AMD. Owing to the rapidly aging population, the number of persons having AMD will increase by 50% to 2.95 million in 2020[1]. In another study, AMD was reported to account for 54% of all current cases of blindness among the Caucasian population in the United States[2]. The study predicted that the number of blind people in the US could increase by as much as 70% by 2020.
Traditional Chinese Medicine, Ligusticum Wallichii Franchat (Chuan Xiong), has been used to treat neurovascular and cardiovascular diseases. TMP is one of the most active ingredients of Chuan Xiong. TMP can increase retinal and choroidal blood flow by 44% and did not affect systemic blood pressure and heart rate on rabbit at the same time[3]. Intraperitoneal administration of TMP on Brown Norway rats could significantly decrease the intensity of fluorescein leakage from the photocoagulated lesions and the size of choroidal neovascularization(CNV) induced by laser treatment[4]. TMP could protect the heart from lipid peroxidation-induced heart toxicity by its prominent anti-lipid peroxidation and anti-free radical formation effects[5]. This study is to observe the effects of Tetramethylpyrazine (TMP) on sodium iodate (NaIO3) induced retinal pigment epithelium (RPE) degeneration, choroidal blood flow in vivo and oxidative stress of RPE cells in vitro.
MATERIALS AND METHODS
Materials
TMP (purity≥98%), NaIO3 (purity≥99.5%), thiazolyl blue tetrazolium bromide (MTT, purity≥97.5%), hydrogen peroxide (50wt% solution in water), tert-Butyl hydroperoxide (t-BHP, 70wt% in water), dimethyl sulfoxide (DMSO, purity≥99.9%), sodium azide (purity≥99.5%), and Dulbecco's modified Eagles's medium/Ham's F12 (DMEM/F12, 1:1) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from GIBCO (Grand Island, NY, USA).
Methods
ERG in rats
A total of thirty 8-week-old male Brown-Norway (BN) rats were randomly divided into 3 groups, 10 rats in normal group, 10 rats in NaIO3 group and 10 rats in TMP+ NaIO3 group. Control group was instilled with solvent(2-hydroxypropyl-β-cyclodextrin, Sigma-Aldrich) alone without NaIO3 injection. NaIO3 group was instilled with solvent plus 35mg/kg NaIO3 injection through hypoglossal vein, whereas TMP+ NaIO3 group was instilled with 1%TMP eye drops plus 35mg/kg NaIO3 injection[6],[7]. Both eyes of all rats were instilled with 1 drop for 3 times a day for 1 week before and 4 weeks after NaIO3 injection[8]. At the end of 2 and 4 weeks, RPE function was measured with c-wave of ERG.
BN rats were dark adapted for 2 hours, and then anesthetized with ketamine 35mg/kg plus xylazine 5mg/kg i.m. Half of the initial dose was given each 1 hour thereafter. Pupils of all rats were dilated with one drop each of 1% atropine, and 25g/L phenylephrine. Before recording, one drop of 5g/L tetracaine was given for surface anesthesia. All rats were kept warm during ERG measurement. DC-ERG recording methods developed by Peachey were followed. Briefly, a 1-mm diameter glass capillary tube with filament (Sutter Instruments, Novato, CA) that was filled with Hanks balanced salt solution (Invitrogen, Carlsbad, CA) was used to connect with a Ag/AgCl wire electrode with a attached connector. The electrode was positioned to the corneal surface. Responses were amplified (dc-100 Hz; gain=1000X; DP-31, Warner Instruments, Hamden,CT) and digitized at 10Hz or 1000Hz. Data were analyzed by iWORX LabScribe Data Recording Software (iWorx0CB Sciences, Dover, NH). Light stimuli was derived from an optical channel using a fiber-lite high intensity illuminator (Dolan-Jenner Industries, Inc.MA), with neutral density filters (Oriel, Stratford, CT) placed in the light path to adjust stimulus luminance. The stimulus luminance used in this experiment was 3.22 log cd/m2 and stimulated for 4 minutes. Luminance calibration was made by a Minolta (Ramsey, NJ) LS-110 photometer focused on the output side of the fiber optic bundle where the rat eye was located.
Measurement of Choroid Blood Flow in Ocular Hypertensive Rabbit Eyes
New Zealand white rabbits, weighing 2.5-3.0kg, were purchased through LARR (Texas A&M University, USA). All rabbits were anesthetized with 35mg/kg ketamine and 5mg/kg xylazine i.m. Half of the initial dose was given each 1 hour thereafter. An ocular hypertensive model was created by raising the intraocular pressure of the left eye to 40mmHg, which reduced the ocular blood flow to approximately one third of the normal valued[9]. The left ventricle was cannulated through the right carotid artery for the injection of colored microspheres and the femoral artery was cannulated for blood sampling. One percent drug solution (50µL) or vehicle (50µL) was instilled topically to the left eye, and the ocular blood flow of the ocular hypertensive rabbits was measured with colored microspheres at 0, 30, 60 and 120 minutes thereafter. At each time point, 2 million microspheres in 0.2mL were injected as a reference, and blood samples were taken from the femoral artery for exactly 1 minute following injection of the microspheres. The blood sample was collected in a heparinized tube and the volume was recorded. The rabbits were euthanized with an injection of 100mg/kg pentobarbital sodium after the last blood sampling. The left eyes were enucleated and dissected into the retina, choroid, iris and ciliary body. The tissue samples were weighed.
The details of sample processing and microsphere counting were provided by E-Z Trac (Los Angeles, CA). In brief, a hemolysis reagent was added to the microfuge tubes with the blood sample, then vortexed and centrifuged for 30 minutes at 6 000r/min. The supernatant was removed, and tissue/blood digest reagents I and II were added. The tubes were capped, vortexed, and centrifuged for 30 minutes again. The supernatant was removed, and the counting reagent was added, then vortexed, and centrifuged for 15 minutes at the same resolutions as above. The supernatant was removed, and the microspheres were resuspended in a precise volume of the counting reagent, The number of microspheres was counted with a hemocytometer.
Tissue/blood digest reagent I was added to the microfuge tubes with the tissue samples, sealed, and heated at 95°C for 15 minutes. The tubes were vortexed for 30 seconds, then reheated and revortexed until all tissue samples were dissolved. The tissue/blood digest reagent II was added while the tissue samples were still hot, then the tubes were capped, vortexed, and centrifuged for 30 minutes. The protocol thereafter was the same as that used to process the blood sampled, and the microspheres were counted.
The blood flow of each tissue at a certain time point was calculated from the following equation:Qm = (Cm × Qr)/Cr in which Qm is the blood flow of a tissue in terms of µL/minute per mg, Cm is the microsphere count per mg of tissue, Qr is the flow rate of blood sample in terms of µL per minute, and Cr is the total microsphere count in the referenced blood sample.
Oxidative Stress of RPE Cells
The hRPE cells line, ARPE-19 (ATCC, Manassas, VA, USA), was used during all experiments. The cells were grown in DMEM/F12 supplemented with 10% FBS, 100units/mL penicillin G, and 100µg/mL streptomycin sulfate. Cells were incubated in a humidified incubator at 37°C under 5% CO2 and 95% air.
The effect of TMP and oxidizing agents on the cell proliferation was evaluated with the MTT assay. RPE cells (8×104 cells) were seeded in 96-well plates (100µl/well) and allowed to grow overnight. Blanks were formed by adding 100µL medium (ODblank). The cells were then treated with fresh medium with TMP and/or oxidizing agents at the same time for 12, 24, or 48 hours (200µl/well, ODcompound). The vehicle control group (ODcontrol) was treated with 1.25‰ DMSO and/or PBS. The medium was then replaced with fresh medium containing 0.5mg/mL MTT but free FBS for 4 hours, 100µl/well. After incubation, the medium was discarded and 150µL DMSO was added to solubilize formazan produced from MTT by the viable cells. Absorbance was measured at 570nm using a microplate reader (Packard BioScience Co. Meriden, CT, USA). Cell viability was calculated according to the following formula: Viability of cell (%) = (ODcompound - ODblank)/(ODcontrol - ODblank)×100%
RPE cells were allowed to attach overnight, and then exposed to TMP and vehicle under hypoxic condition for 24 hours. Hypoxic conditions (1% O2 and 5% CO2) were maintained by using a temperature- and humidity-controlled environmental C-chamber by O2 and CO2 controllers (Proox Model 110 and Pro CO2 Model 120, Bio Spherix Ltd., Redfield, NY) with N2 and CO2 gas sources. Hypoxic culture medium was pre-equilibrated overnight prior to cell exposure.
Statistical Analysis
All data were presented as mean±SEM. A nonpaired Student's t-test was performed to analyze the significance between two means at a certain time point. The differences were considered significant at P<0.05.
RESULTS
ERG Recordings
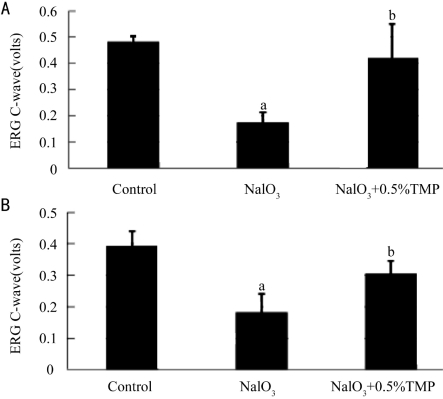
ERG c-wave of rats was measured at 2 and 4 weeks after administration of 35mg/kg NaIO3. It was found that 2 weeks after NaIO3 injection (Figure 1A), the amplitude of ERG c-wave of NaIO3 group fell markedly to 36% of control group (P<0.01). No significant change was observed in TMP+NaIO3 group as compared to control. Four weeks after NaIO3 administration (Figure 1B), the ERG c-wave of NaIO3 group fell to 46% of control group (P<0.01) whereas the TMP+NaIO3 group only fell to 77% of control group (P<0.01). There was a significant reversal of NaIO3 group by TMP (Control: 0.393±0.046 millivolts; NaIO3:0.183±0.058 millivolts; TMP+NaIO3: 0.305±0.041 millivolts, P<0.01).
Figure 1. A: effect of TMP on NaIO3 induced RPE degeneration in rat eyes for 2 weeks. a: control group compares with NaIO3 group (P=4.83E-9). b: NaIO3+0.5%TMP group compares with NaIO3 group(P=9.78E-5); B: effect of TMP on NaIO3 induced RPE degeneration in rat eyes for 4 weeks. a: control group compares with NaIO3 group(P=8.98E-6). b: NaIO3+0.5%TMP group compares with NaIO3 group(P=3.92E-5).
Choroid Blood Flow in Ocular Hypertensive Rabbit Eyes
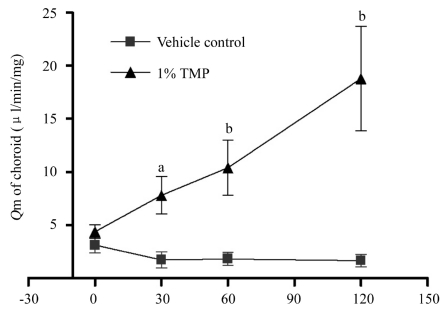
The choroid blood flow declined gradually in the vehicle control group. TMP significantly increased the choroid blood flow at time 30, 60 and 120 minutes as compared with the vehicle control group (Figure 2).
Figure 2. Effect of TMP on the choroid blood flow of rabbit eyes.
aP<0.05 and bP<0.01 vs vehicle control group. The data was expressed as mean±SEM, in the vehicle control group n =7 and in the TMP group n =6
Effect of TMP on Oxidants-Induced Damage in RPE Cells
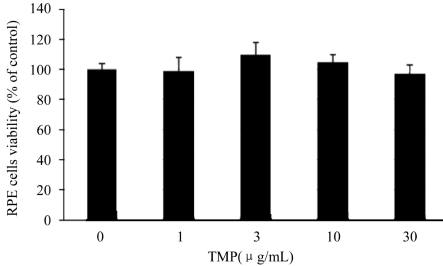
TMP, up to the concentration of 30µg/mL, did not inhibit the proliferation of RPE cells (Figure 3). The result indicated that TMP itself had no toxicity on RPE cells at the concentration used in the experiments.
Figure 3. Effect of TMP on the proliferation of RPE cells.
RPE cells were incubated with TMP for 48 hours
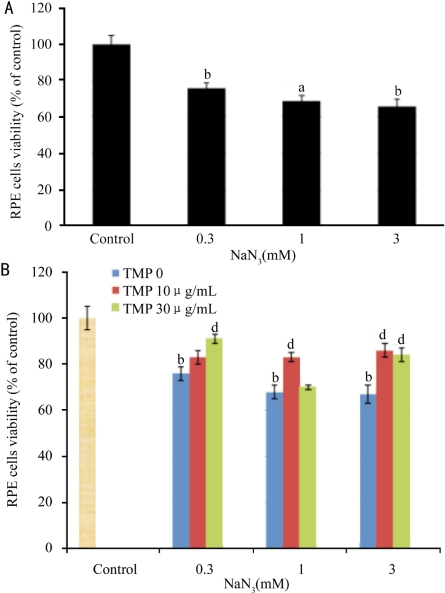
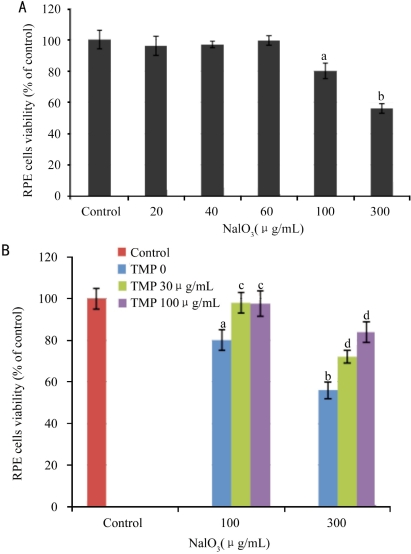
TMP has no effect on hypoxia, t-BHP, and H2O2-induced injury in RPE cells (data not shown). However, TMP reversed NaN3-induced injury in RPE cells (Figure 4). 10µg/mL TMP reversed 1 and 3mM NaN3-induced loss of viability of RPE by 18.5% (P<0.01) and 23.0% (P <0.01), respectively. 30µg/mL TMP reversed 0.3 and 3 mM NaN3-induced loss of viability of RPE from 73.8% to 91.0% (P<0.01) and from 64.7% to 83.6% (P<0.01), respectively. NaIO3 concentration-dependently reduced the viability of RPE cells. 100µg/mL NaIO3 significantly inhibited the proliferation of RPE cells (P<0.05, Figure 5A). TMP significantly reversed NaIO3-induced injury in RPE cells (Figure 5B). 30µg/mL TMP reversed 30 and 100 mM NaIO3-induced loss of viability of RPE by 18.1% (P<0.05) and 16.8% (P<0.01), respectively. 100µg/mL TMP reversed 30 and 100 mM NaIO3-induced loss of viability of RPE from 80.0% to 97.3% (P<0.05) and from 55.5% to 84.2% (P<0.01), respectively.
Figure 4. Effect of TMP on NaN3-induced ischemia toxicity in RPE cells.
A: effect of NaN3 on the proliferation of RPE cells; B: Effect of TMP on NaN3-induced ischemia toxicity in RPE cells. RPE cells were incubated with TMP and NaN3 for 48 hours. aP<0.05 and bP<0.01 vs vehicle control. dP<0.01 vs model control. The data was expressed as mean±EM, n=6
Figure 5. Effect of TMP on NaIO3-induced injury in RPE cells.
A: effect of NaIO3 on the proliferation of RPE cells; B: effect of TMP on NaIO3-induced injury in RPE cells. RPE cells were incubated with TMP and NaIO3 for 48 hours. aP<0.05 and bP<0.01 vs vehicle control. cP<0.05 and dP<0.01 vs model control. The data was expressed as mean±SEM, n =6
DISCUSSION
AMD, is divided into wet-AMD and dry-AMD, and is a debilitating disease of the eye, which manifests clinically with loss of central vision and pathologically with the accumulation of drusen, RPE degeneration, photoreceptor atrophy, and in wet AMD cases, with CNV formation. There are several risk factors, including age, race, smoking, and diet[10]. But the etiology and pathogenesis of the disease remain largely unclear. For wet-AMD, there are a number of established treatment options, such as laser photocoagulation, intravitreal corticosteroids, verteporfin photodynamic therapy (V-PDT), anti-angiogenic factors, surgery, and combination of several treatments[11]. Most optional treatments are for the wet-AMD, not for dry-AMD. There is no effective treatment today for the most prevalent dry- AMD[12].
Dry-AMD is triggered by abnormalities in RPE that lies beneath the photoreceptor cells and normally provides critical nutritional and metabolic support to these light-sensing cells. Secondary to RPE dysfunction, macular rods and cones degenerate leading to the irreversible loss of vision. Oxidative stress, formation of drusen, accumulation of lipofuscin, local inflammation and reactive gliosis represent the pathologic processes implicated in pathogenesis of dry-AMD. The direct toxic effect of NaIO3 on RPE cells with secondary effects on photoreceptors and the choriocapillaries in vivo is well known[13]. The mechanisms of the toxicity of NaIO3 to RPE cells are as follows: first, NaIO3 can increase the ability of melanin to convert glycine into glyoxylate, a potential cell toxic compound[14]; second, NaIO3 could denaturant retinal proteins by changes of -SH levels in retina[15]; third, NaIO3 could cause considerable structure changes by breakdown of RPE diffusion barrier or by reduction of adhesion between RPE and photoreceptor cells[16]-[19]; finally, NaIO3 inhibits various enzyme activities, such as triose phosphate dehydragenase, succinodehydrogenase and lactate dehydrogenase[18],[20].
The ERG results showed that TMP can reverse NaIO3-induced injury in RPE cells by 67% at the end of 4 weeks. TMP showed protective effect against NaIO3-induced RPE degeneration in rat eyes. The NaIO3 intoxication causes death of RPE cells and photoreceptor damage followed by marked phagocytic activity of proliferating de-differentiated pigmental cells leading to the final pigmentary picture of the fundus. Further morphological study is needed to reveal the TMP role in RPE protection. Our previous study showed that TMP could significantly decrease the intensity of fluorescein leakage from the photocoagulated lesions and the size of CNV induced by laser treatment on Brown Norway rats, and interfered with vascular endothelial cell proliferation in vitro[4].
The choroid blood flow results showed significant increase of choroid blood flow by TMP. The increase of choroid blood flow may facilitate removal of metabolic wastes and replenish nutrients to RPE and photocells, thus it may change the microenviroment, change the balance between pro- and antiangiogenesis factors, and then the change of the process of angiogenesis.
Mitochondria are the powerhouse of the cell, and their primary function is to generate ATP through oxidative phosphorylation via the electron transport chain[21]. NaN3 is an inhibitor of cytochrome oxidase and catalase, and will downregulate electron transport and O2 consumption to cause the death of cells[22]. TMP could reverse NaN3-induced injury in RPE cells (Figure 4), but have no effect on hypoxia-induced damage (1% O2) in RPE cells. The results indicated that TMP can reverse NaN3-induced ischemia by protecting cytochrome oxidase and/or catalase. Our experiments also showed that TMP reversed NaIO3-induced injury in RPE cells in vitro (Figure 5).
In conclusion, TMP might slow the oxidative process of RPE cell layer which leads to RPE degeneration. TMP might also prevent the formation of CNV. According to the rational of the AMD, the RPE abnormalities or degeneration is the key point of both dry and wet AMD, so TMP could be used to prevent and treat both dry and wet AMD in the future.
REFERENCES
- 1.Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Chiou GC, Yan HY, Lei XL, Li BH, Shen ZF. Ocular and cardiovascular pharmacology of tetramethylpyrazine isolated from Ligusticum wallichii Franch. Zhongguo Yaoli Xuebao. 1991;12(2):99–104. [PubMed] [Google Scholar]
- 4.Zou YH, Jiang W, Chiou GC. Effect of tetramethylpyrazine on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. Curr Eye Res. 2007;32(1):71–75. doi: 10.1080/02713680601088787. [DOI] [PubMed] [Google Scholar]
- 5.Liu CF, Lin CH, Chen CF, Huang TC, Lin SC. Antioxidative effects of tetramethylpyrazine on acute ethanol-induced lipid peroxidation. Am J Chin Med. 2005;33(6):981–988. doi: 10.1142/S0192415X05003570. [DOI] [PubMed] [Google Scholar]
- 6.Grignolo A, Orzalesi N, Calabria GA. Studies on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by sodium iodate. Exp Eye Res. 1966;5(1):86–97. doi: 10.1016/s0014-4835(66)80024-6. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, Chiou GC. Effects of hydralazine on NaIO3-induced rat retinal pigment epithelium degeneration. Int J Ophthalmol. 2008;8(8):1504–1510. [Google Scholar]
- 8.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57(14):2010–2032. doi: 10.1016/j.addr.2005.09.004. Epub 2005 Nov 10. [DOI] [PubMed] [Google Scholar]
- 9.Chiou GC, Chen YJ. Effects of D- and L-isomers of timolol on retinal and choroidal blood flow in ocular hypertensive rabbit eyes. J Ocul Pharmacol. 1992;8(3):183–190. doi: 10.1089/jop.1992.8.183. [DOI] [PubMed] [Google Scholar]
- 10.Park YH, Chiou CY. Structure-activity relationship (SAR) between some natural flavonoids and ocular blood flow in the rabbit. J Ocul Pharmacol Ther. 2004;20(1):35–42. doi: 10.1089/108076804772745446. [DOI] [PubMed] [Google Scholar]
- 11.Peachey NS, Stanton JB, Marmorstein AD. Noninvasive recording and response characteristics of the rat dc-electroretinogram. Vis Neurosci. 2002;19(6):693–701. doi: 10.1017/s0952523802196015. [DOI] [PubMed] [Google Scholar]
- 12.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002;25(6):373–379. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- 14.Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992;5(6):394–395. doi: 10.1111/j.1600-0749.1992.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 15.Sorsby A, Reading HW. Experimental degeneration of the retina. XI. The effect of sodium iodate on retinal -SH levels. Vis Res. 1964;4(10):511–514. doi: 10.1016/0042-6989(64)90057-4. [DOI] [PubMed] [Google Scholar]
- 16.Flage T, Ringvold A. The retinal pigment epithelium diffusion barrier in the rabbit eye after sodium iodate injection. A light and electron microscopic study using horseradish peroxidase as a tracer. Exp Eye Res. 1982;34(6):933–940. doi: 10.1016/0014-4835(82)90072-0. [DOI] [PubMed] [Google Scholar]
- 17.Sen HA, Berkowitz BA, Ando N, de Juan E Jr. In vivo imaging of breakdown of the inner and outer blood-retinal barriers. Invest Ophthalmol Vis Sci. 1992;33(13):3507–3512. [PubMed] [Google Scholar]
- 18.Ashburn FS, Jr, Pilkerton AR, Rao NA, Marak GE. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19(12):1427–1432. [PubMed] [Google Scholar]
- 19.Stern WH, Ernest JT, Steinberg RH, Miller SS. Interrelationships between the retinal pigment epithelium and the neurosensory retina. Aust J Ophthalmol. 1980;8(4):281–288. doi: 10.1111/j.1442-9071.1980.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82(3):441–448. doi: 10.1016/j.exer.2005.08.002. Epub 2005 Sep 19. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol Sin. 2007;28(3):315–326. doi: 10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118(1):47–55. [PubMed] [Google Scholar]