Abstract
AIM
To study the effects of naringenin eye drops on NaIO3-induced retinal pigment epithelium (RPE) degeneration and laser-induced choroidal neovascularization (CNV) in rat eyes.
METHODS
The 35mg/kg NaIO3-induced RPE degeneration was prevented by 10g/L naringenin eye drops 3 times a day for 7 days in advance of NaIO3 injection, and then 2 to 4 weeks thereafter, RPE function was measured with C-wave of electroretinogram (ERG). The laser-induced CNV rats were treated with laser to break the Bruch's membrane and the CNV formation was prevented by 10g/L naringenin eye drops instilled 3 times a day for 2 to 4 weeks. The CNV formation was measured with fluorescein angiography (FA) and flat mount.
RESULTS
Two weeks after NaIO3 injection, the amplitude of ERG C-wave fell markedly in NaIO3 group to 53% of normal group (P<0.01). No apparent difference was observed in naringenin+NaIO3 group. Four weeks later, the NaIO3 group fell to 37% of normal group (P<0.01), while the naringenin+ NaIO3 group fell to only 57% of normal group (P<0.01). There was a 52% reversal of the ERG C-wave by naringenin as compared to NaIO3 treated group (P<0.05). Two weeks and four weeks after laser treatment, naringenin reduced the CNV formation to 53% and 49% of control group (100%) measured by FA (P<0.01). Four weeks after laser treatment, naringenin reduced the CNV formation by 47% as compared to control group measured with flat mount (P<0.01).
CONCLUSION
Naringenin can significantly protect RPE from NaIO3 induced degeneration and also prevent CNV formation.
Keywords: naringenin, sodium iodate, laser, retinal pigment epithelium, age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD) was first described in the medical literature in 1875 as “symmetrical central choroidoretinal disease occurring in senile persons”[1]. It was not until 1980 that macular degeneration was reported to be a significant cause of blindness in the United States[2]. A 2004 analysis reported that among Americans over the age of 40, AMD and/or geographic atrophy were present in at least one eye in 1.47% of the population, and that 1.75 million individuals have AMD[3]. By the year 2020, there may be a 50% increase in the incidence of AMD. In another study, AMD was reported to account for 54% of all current cases of blindness among the Caucasian population in the United States[4]. The study predicted that as a result of the rising prevalence of AMD, the number of blind people in the US could increase by as much as 70% by 2020. Naringenin, a natural predominant flavanone, has a wide range of pharmacologic activities. In our previous study, it was found to improve ocular blood flow and cause retinal function recovery both in rabbits and rats[5]. In this study, we observe the effect of naringenin on NaIO3- and laser- induced AMD.
MATERIALS AND METHODS
Materials
Eight-week-old male Brown-Norway (BN) rats were purchased through LARR (Texas A&M University, USA). All rats had free access to a standard diet and drinking water and were housed in a standard animal room maintained at 24±0.5°C, with relative humidity of 14%±5%, and with a 12:12 hours cyclic lighting schedule. All of the procedure conformed to the ARVO Resolution on the use of animals in ophthalmic and vision research. NaIO3 (Sigma-Aldrich) was dissolved in saline at a mass concentration of 30g/L. Injection of 35mg/kg NaIO3 was made through hypoglossal vein. Naringenin (Sigma-Aldrich) was dissolved by 230g/L 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) to 10g/L concentration. Ten percent fluorescein sodium salt (Sigma-Aldrich) was injected through hypoglossal vein at 0.5mL/kg. Ten percent fluorescein isothiocyanate-dextran (Sigma-Aldrich) was injected through hypoglossal vein at 1.4mL/kg after 3 days of fluorescein sodium salt injection.
Methods
Animal procedure
A total of 26 rats were randomly divided into three groups, 5 rats in normal group, 10 rats in NaIO3 group and 11 rats in naringenin+NaIO3 group. Normal group was instilled with solvent alone without NaIO3 injection. NaIO3 group was instilled with solvent alone after 35mg/kg NaIO3 injection, whereas naringenin+NaIO3 group was instilled with 10g/L naringenin eye drops after 35mg/kg NaIO3 injection. All eye drops were instilled 3 times a day for 1 week before and 4 weeks after NaIO3 injection. At the end of 2 and 4 weeks, all rats were measured C-wave with ERG. Another 20 rats were divided into two groups, 10 for control group and another 10 for the naringenin group. Control group was instilled with solvent. Naringenin group was instilled with 10g/L naringenin eye drops. All eye drops were instilled 3 times a day for 1 week before and 4 weeks after laser treatment. At the end of 2 and 4 weeks, all rats were measured with FA.
ERG recording
BN rats were dark adapted for 2 hours, and then anesthetized with ketamine 35mg/kg plus xylazine 5mg/kg im. Half of the initial dose was given each 1 hour thereafter. Pupils of all rats were dilated with one drop each of 10g/L atropine, and 25g/L phenylephrine. Before recording, one drop of 5g/L tetracaine was given for surface anesthesia. All rats were kept warm during ERG measurement. DC-ERG recording methods developed by Dr. Peachey were followed. Briefly, a 1mm diameter glass capillary tube with filament (Sutter Instruments, Novato, CA) that was filled with Hanks balanced salt solution (Invitrogen, Carlsbad, CA) was used to contact with a Ag/AgCl wire electrode with an attached connector[6]. The capillary tube was connected with rat's corneal surface completely. Another similar electrode placed on the surface of the other eye served as a reference lead. Responses were amplified(dc-100Hz;gain=1000X; DP-31,Warner Instruments, Hamden, CT) and digitized at 10Hz or 1000Hz. Data were analyzed by iWORX LabScribe Data Recording Software (iWorx0CB Sciences, Dover, NH). Light stimuli were derived from an optical channel using a fiber-lite high intensity illuminator (Dolan-Jenner Industries, Inc. MA), with neutral density filters (Oriel, Stratford, CT) placed in the light path to adjust stimulus luminance. The stimulus luminance used in this experiment was 3.22 log·cd/m2 and stimulated for 4 minutes. Luminance calibration was made by a Minolta (Ramsey, NJ) LS-110 photometer focused on the output side of the fiber optic bundle where the rat eye was located.
Laser-induced CNV
BN rats were anesthetized with ketamine 35mg/kg plus xylazine 5mg/kg im. Pupils were dilated with one drop each of 10g/L atropine, plus 25g/L phenylephrine. Ocular fundus was visualized with a VOLK super pupil XL biomicroscopy lens (Keeler Instrument Inc., Broomall, PA). Double-frequency Nd:YAG laser (Laserex LP3532; Lumenis Inc., Salt Lake City, UT) was used at 532-nm wavelength. Laser parameters used were 100-µm spot size, 0.15-second exposure and 150-200mW powers. Five laser spots were made to the ocular fundus at approximately equal distances around the optic nerves. Only laser spots with bubble formation were included in the study. Lesions with subretinal hemorrhage were excluded.
Fluorescein angiography
FA was performed after 2 and 4 weeks laser treatment with a digital fundus camera (TRC-50EX; TOPCON, Tokyo, Japan). Ten percent fluorescein sodium salt was injected through hypoglossal vein at 0.5mL/kg. Both early (under 2 minutes) and late (over 7 minutes) fluorescein phases were captured. Ten percent fluorescein isothiocyanate-dextran was injected through hypoglossal vein at 1.4mg/kg after 3 days of fluorescein sodium salt injection. Fluorescein pictures were taken within 20 minutes. The clearest pictures were chosen for the areas of CNV formation measurement by Imagenet2000 digital imaging system (Topcon Medical Systems, Inc., Paramus, NJ).
Choroidal flat mount
After FA taken, all rats were sacrificed at the end of 4 weeks. The eye balls were enucleated and put in Glutaraldehyde solution (Grade 1, 25%). The cornea, lens, vitreous body and the retina were removed. And the eyecup was flat mounted with the choroids facing up. Flat mounts were captured by fluorescence microscopy on an Axioskop microscope (Zeiss, Thornwood, NY) and Image-Pro Plus software (Media cybernetics, Silver Spring, MD) was used to measure the area of CNV.
Statistical Analysis
Both eyes of each rat were measured in the experiments. All data were presented as mean±SD. A non-paired Student's t-test was performed to analyze the significance between two means at a certain time point. The differences were considered significant at P<0.05.
RESULTS
ERG Recordings
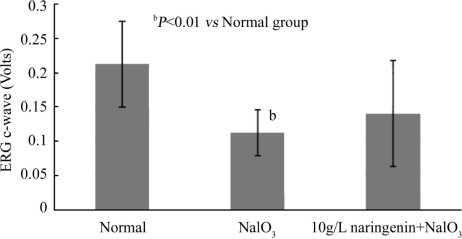
ERG C-wave of rats was measured at 2 and 4 weeks after administration of 35mg/kg NaIO3. It was found that 2 weeks after NaIO3 injection (Figure 1), the amplitude of ERG C-wave fell markedly in NaIO3 group to 53% of normal group (P <0.01). No significant change was observed in naringenin+NaIO3 group as compared to control.
Figure 1. Effect of naringenin on NaIO3-induced RPE degeneration in rat eyes for 2 weeks.
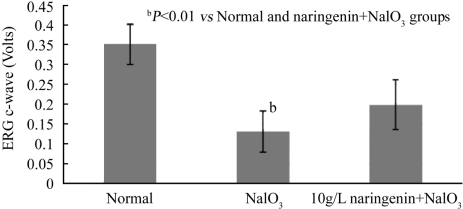
Four weeks after NaIO3 administration (Figure 2), the ERG C-waves of both NaIO3 group and naringenin+NaIO3 group fell further. The ERG C-wave of NaIO3 group fell to 37% of normal group (P<0.01) while the naringenin+NaIO3 group only fell to 57% of normal group (P<0.01). There was a significant reversal by 52% of NaIO3 treated group by naringenin (Normal:0.351±0.052 volts; NaIO3:0.131±0.052 volts; naringenin+ NaIO3: 0.199±0.063 volts, P<0.05).
Figure 2. Effect of naringenin on NaIO3-induced RPE degeneration in rat eyes for 4 weeks.
Fluorescein Angiography
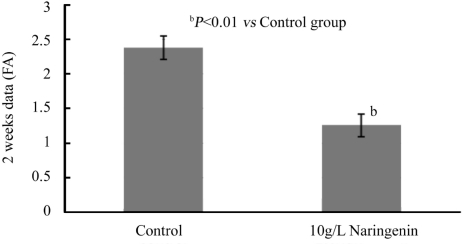
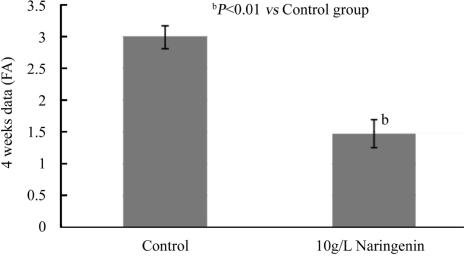
After 2 weeks and 4 weeks treatment, naringenin+laser group reduced the area of CNV significantly as compared to control group (Figures 3,4). Two weeks after laser treatment, naringenin reduced the CNV formation by 47% measured with FA (Normal:2.377±0.171mm2; Naringenin:1.259±0.161mm2, P<0.01). Four weeks after laser treatment, naringenin reduced the CNV formation by 51% as compared to control group (Normal:2.985±0.181mm2; Naringenin:1.467±0.215mm2, P<0.01).
Figure 3. Effect of naringenin eye drops on laser-induced AMD in rat eyes for 2 weeks.
Figure 4. Effect of naringenin eye drops on laser-induced AMD in rat eyes for 4 weeks.
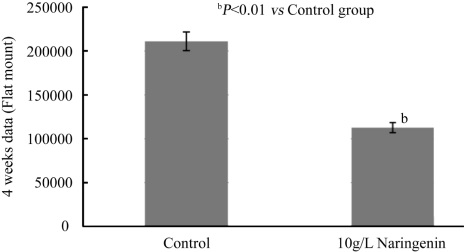
Choroidal Flat Mount
Four weeks after laser treatment, naringenin reduced the CNV formation by 47% as compared to control group measured with flat mount (Normal:211008±10654µm2; Naringenin: 112821±5691µm2, P <0.01, Figure 5).
Figure 5. Effect of naringenin eye drops on laser-induced AMD in rat eyes for 4 weeks.
DISCUSSION
AMD, is divided into wet-AMD and dry-AMD, and is a debilitating disease of the retina, which manifests clinically with loss of central vision and pathologically with the accumulation of drusen, RPE degeneration, photoreceptor atrophy, and in wet AMD cases, with CNV formation. There are several risk factors, including age, race, smoking, and diet[7]. But the etiology and pathogenesis of the disease remain largely unclear. For wet-AMD, there are a number of established treatment options, such as laser photocoagulation, intravitreal corticosteroids, verteporfin photodynamic therapy (V-PDT), anti-angiogenic factors, surgery, and combination of several treatments[8]. Laser photocoagulation prevented the rapid spread of CNV, but often led to some degree of permanent vision loss. V-PDT and anti-angiogenic agents (VEGF inhibitors) arrested the progress of the disease and even improved visual acuity for a small percentage of patients, but the high price is a big problem, especially in the developing country.
Dry-AMD is triggered by abnormalities in the RPE that lies beneath the photoreceptor cells and normally provides critical metabolic support to these light-sensing cells. Secondary to RPE dysfunction, macular rods and cones degenerate leading to the irreversible loss of vision. Oxidative stress, formation of drusen, accumulation of lipofuscin, local inflammation and reactive gliosis represent the pathologic processes implicated in pathogenesis of dry-AMD. But most optional treatments are for the wet-AMD, not for dry-AMD. There is no effective treatment today for the most prevalent dry-AMD[9]. According to the result, after 2 weeks and 4 weeks treatment, 10g/L naringenin could reduce the CNV formation by 47% and 51% as compared to control group. It means 10g/L naringenin may prevent the formation of CNV or slow down the rapid spread of CNV, and also means 10g/L naringenin may stop the dry-AMD developing to wet-AMD.
Our previous studies showed that naringenin might improve ocular blood flow and cause retinal function recovery both in rabbits and rats, and protect RPE cells from degeneration when cultured with NaIO3[5],[10]. In this experiment, two weeks and 4 weeks after injection, NaIO3 caused obvious damage to retinal function in NaIO3 group. No apparent change was observed in naringenin+ NaIO3 group in the first 2 weeks. However 4 weeks later, the ERG C-wave in naringenin+ NaIO3 group didn't fall as markedly as in NaIO3 group. This result might indicate that naringenin had protective effect against NaIO3 induced RPE degeneration in rat eyes and might slow down the development of dry-AMD.
In conclusion, Naringenin might slow the oxidative process of RPE cell layer which leads to RPE degeneration. Naringenin might also prevent the formation of CNV. Thus it might be used to treat both dry and wet AMD in the future. Future study is warranted.
REFERENCES
- 1.Hutchison W. Tay. Symmetrical central choroidoretinal disease occurring in senile persons, R. Lond. Ophthal Hosp Rep. 1875;8:231–244. [Google Scholar]
- 2.Leibowitz HM, Krueger DA, Maunder RA. An ophthalmological study of cataract, glaucoma, diabetic retinopathy, macular degeneration and visual acuity in a general population of 2631 adults 1973-1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- 3.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Park YH, George C. Y. Chiou. Structure-activity relationship (SAR) between some natural flavonoids and ocular blood flow in the rabbit. J Ocul Pharmacol Ther. 2004;20(1):35–42. doi: 10.1089/108076804772745446. [DOI] [PubMed] [Google Scholar]
- 6.Peachey NS, Stanton JB, Marmorstein AD. Noninvasive recording and response characteristics of the rat dc-electroretinogram. Vis Neurosci. 2002;19(6):693–701. doi: 10.1017/s0952523802196015. [DOI] [PubMed] [Google Scholar]
- 7.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin Albert J, Stefan Scholl, Kirchhof J. Treatment of neovascular age-related macular degeneration: Current therapies. Clin Ophthalmol. 2009;3:175–182. doi: 10.2147/opth.s3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opin Ther Targets. 2007;11(5):625–639. doi: 10.1517/14728222.11.5.625. [DOI] [PubMed] [Google Scholar]
- 10.Baoqin Lin, Chiou George C.Y. Antioxidant activity of naringenin on various oxidants induced damages in ARPE-19 cells and HUVEC. Int J Ophthalmol (Guoji Yanke Zazhi) 2008;8(10):1963–1967. [Google Scholar]