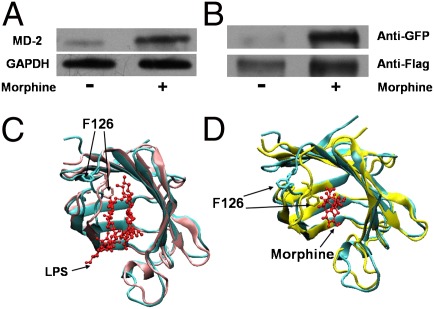
Fig. 2.
Morphine induces TLR4 oligomerization and activates TLR4 signaling. (A) Morphine bound to MD-2 in a cellular environment. HEK Blue hTLR4 cells, which overexpress the human TLR4 and MD-2, were treated with morphine (300 μM) for 12 h, and cell lysates were immunoprecipitated by morphine antibody and then detected by Western blotting by MD-2 antibody. GAPDH served as the cell lysates input control. (B) Morphine-induced TLR4 receptor oligomerization. Ba/F3 cells simultaneously overexpressing human TLR4-Flag, human TLR4-GFP, human CD14, and human MD-2 were stimulated with morphine (300 μM) for 72 h. Cells were then subjected to immunoprecipitation with anti-Flag antibody and immunoprobing with anti-GFP antibody (Upper) and anti-Flag antibody (Lower). (C and D) Structural comparison of apo- and ligand-bound MD-2. The ligands are shown in a ball-and-stick presentation. X-ray crystal structure of apoMD-2 (cyan; PDB ID 2E56) superimposed with (C) X-ray crystal structure of the LPS-bound MD-2 (pink; PDB ID 3FXI) or (D) morphine-bound MD-2 (yellow) derived from atomic molecular dynamics simulation.