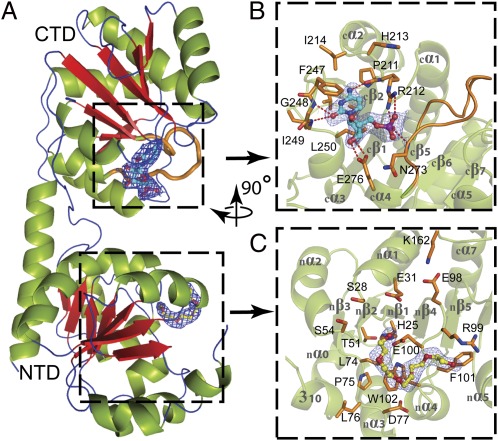
Fig. 1.
Domain organization of WaaAAAE. (A) Overall structure. β-Strands are shown in red, α-helices in green, and loops and turns in blue. (B) Donor-substrate binding site of the C-terminal domain (CTD). Residues forming the CMP-binding site are shown in orange. Dashed lines indicate hydrogen bonds. In A and B the putative Kdo-binding loop (cβ5–cα4, 263GGTFVNIGGHNLLE276) is highlighted in orange. (C) Putative acceptor-substrate binding site of the N-terminal domain (NTD) occupied by a PEG molecule. Residues potentially involved in acceptor-substrate binding are shown in orange. The final 2|Fo|-|Fc| electron-density maps surrounding the bound CMP and PEG molecules are contoured at 1 σ above the mean. Oxygen atoms are colored red, nitrogen atoms blue, and the phosphorous atom purple. The orientation of B and C with respect to A is indicated by the rotation axis.