Fig. 2.
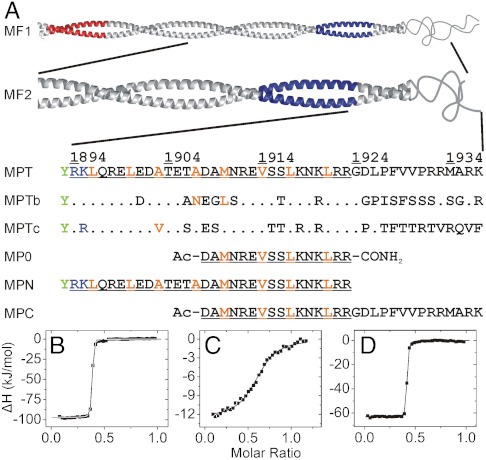
Nonmuscle myosin II heavy chain fragments and peptides used in this work and their affinity to S100A4. (A) Fragments MF1 (NMIIA Ser1712-Glu1960) and MF2 (NMIIA Gln1795-Lys1937) form coiled-coil structures, MF1 assembles into filaments (33). The mainly negatively (Glu1722-Asn1756) and positively charged (Ala1868-Lys1895) regions (the latter called ACD) that are crucial for filament formation (33, 34) colored in red and blue, respectively. Within the sequence of MPT (NMIIA Arg1894-Lys1937) the residues that are thought to form a coiled-coil in longer fragments are underlined, while orange letters show a and d positions in the heptad repeat. Note that the N-terminal basic residues of MPT overlap with ACD and MPT does not contain the full nonhelical tailpiece. Corresponding amino acid positions of S100A4 binding site that differ in NMIIB (1901-1944) and NMIIC (1918-1961) isoforms are indicated. An N-terminal Tyr (green) was added to the peptides MPT and MPN to facilitate concentration measurements. (B–D) Heat changes were recorded by ITC experiments at 25 °C, at physiological salt concentration. 75 μM S100A4 monomer was titrated with 500 μM monomeric NMIIA peptides. Peptides MPN (B) and MPT (D) show nanomolar affinity to S100A4 (Kd < 8 nM), while the binding affinity of MPC (C) is in the micromolar range (Kd ≈ 1.8 μM). In all cases, the binding stoichiometry is approximately one myosin peptide per S100A4 dimer. Thermodynamic parameters of the interactions of S100A4 with NMII heavy chain fragments are shown in Table S1.