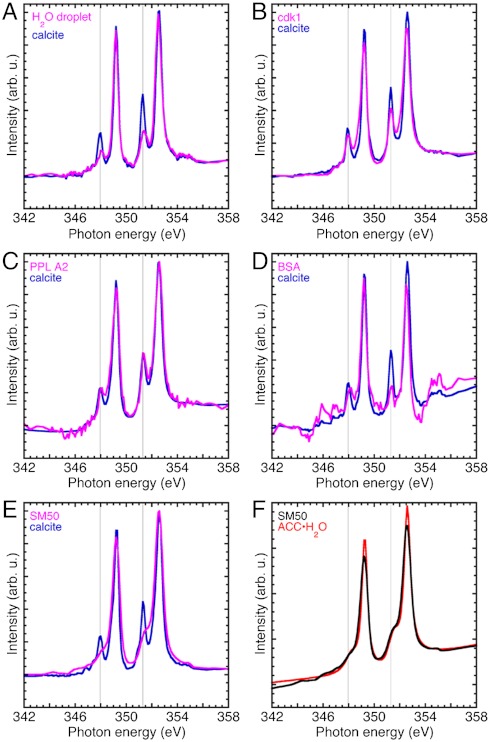
Fig. 4.
Ca L-edge spectra acquired with XANES-PEEM on the surface of single-crystal calcite wafers, after depositing a droplet of water or protein in water, and letting it air dry. All data were acquired at the edge of each dried droplet, thus the two spectra in each plot were acquired simultaneously (magenta and blue curves). (A) The water droplet suspends a few ions or ion clusters, which then reprecipitate as calcite. The lower intensity crystal field peaks (vertical lines) in the H2O-dried droplet spectrum indicate a polycrystalline calcite precipitate. (B) Cdk1 is a yeast cell cycle kinase expressed and extracted with the same methods used for SM50. This control spectrum also shows calcite reprecipitation. (C) PPL A2 is another protein present in sea urchin spicules (11). (D) The BSA dried droplet was very thick, hence the Ca signal is weak and noisy, but clearly calcite. (E) In the presence of the spicule matrix protein SM50 the reprecipitate is not calcite but hydrated ACC. (F) The SM50 spectrum from E, overlapped with the fitted ACC·H2O from Fig. 1B (red curve). The two spectra are very similar. Hence we deduce that SM50 stabilizes ACC·H2O, even in a dried droplet, in ultra-high vacuum, and in direct contact with a crystalline calcite wafer.