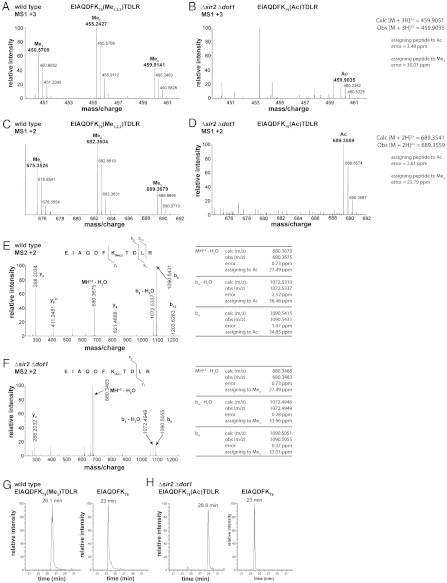
Fig. 7.
Mass spectra of endogenous histone H3 peptides from wild-type and Δsir2 Δdot1 yeast strains. (A) MS1 spectrum of triply charged peaks from wild-type histone H3 peptides shows mono-, di-, and trimethylated forms of H3 K79. (B) MS1 spectrum of triply charged peaks from the sir2 dot1 double knockout. The methylated forms of H3 K79 are absent in this strain; however, a new peak corresponding to the mass of an acetylated form of H3 K79 is observed. (C) MS1 spectrum of doubly charged histone H3 peptides from the wild-type strain. (D) MS1 spectrum of doubly charged histone H3 peaks from the sir2 dot1 double knockout. (E) MS2 spectrum of the doubly charged, trimethylated form of the H3 K79 peptide (EIAQDFK79(Me3)TDLRA) from wild type. (F) MS2 spectra of a doubly charged H3 K79 peptide from the Δsir2 Δdot1 strain. The mass accuracy of the b9 ion of this spectrum confirms that the modification on K79 is acetylation and not trimethylation. (G) Extracted ion chromatogram (XIC) of H3 peptides from wild-type yeast that eluted at an m/z corresponding to the trimethylated K79 peptide, EIAQDFK79(Me3)TDLR (Left). These peptides had a retention time of 26.1 min. The unmodified peptide, EIAQDFK79, eluted at 23 min (Right). (H) XIC of H3 peptides from Δsir2 Δdot1 that eluted at an m/z corresponding to the acetylated K79 peptide, EIAQDFK79(Ac)TDLR (Left). These peptides had a retention time of 28.8 min. The control unmodified peptide, EIAQDFK79 , also eluted at 23 min (Right), as in G.