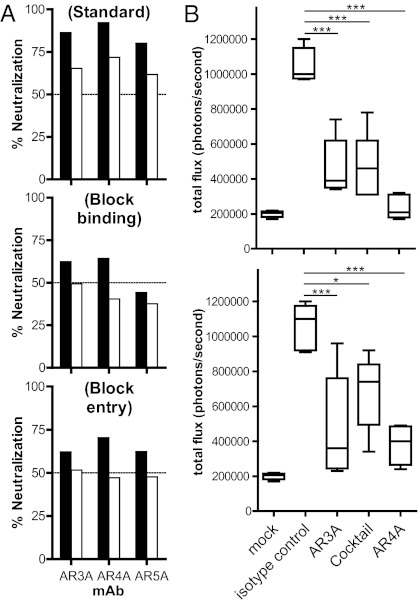
Fig. 2.
(A) The mAbs AR3A, AR4A, and AR5A neutralize HCV at pre- and postattachment steps. In a standard neutralization assay (Top), HCVpp was incubated with the mAbs before spinoculation onto cell monolayers, and the virus was removed after a 6-h incubation at 37 °C. To detect antibody blockage of attachment (Middle), mAbs were incubated with HCVpp before spinoculation onto cells at 8 °C for 1 h. Unbound HCVpp was washed with ice-cold media, and virus infectivity was determined as above. To detect antibody neutralization postattachment (Bottom), HCVpp was first spinoculated onto cells, and unbound virus was removed by washing cell monolayers with ice-cold media before antibodies were added to the cells and infectivity was determined after a 3-d incubation. Black bars, mAbs at 50 μg/mL; white bars, mAbs at 10 μg/mL. (B) Passive antibody protection of a genetically humanized mouse model for HCV infection. Rosa26-Fluc mice (48) (n = 5 for each group) were injected i.p. with 100 mg/kg of mAb AR3A, AR4A, a mixture of half the amount of both mAbs (AR3A/AR4A), or an isotype control IgG1 on days −3 and −2. On day −1, the animals were injected with 1011 recombinant adenoviral particles encoding human CD81, scavenger receptor class B type I (SR-BI), claudin 1, and occludin, and the animals were challenged with 2 × 107 50% tissue culture infectious dose (TCID50) of chimeric HCVcc carrying the structural proteins of the genotype 1b Con1 isolate (BiCRE-Con1/JFH1 virus; Upper) or genotype 2a J6 isolate (BiCRE-Jc1/JFH1 virus; Lower) on day 0. Bioluminescence was detected after 3 d. Data are summarized in box-and-whisker plots, and statistical significance was evaluated by one-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001).