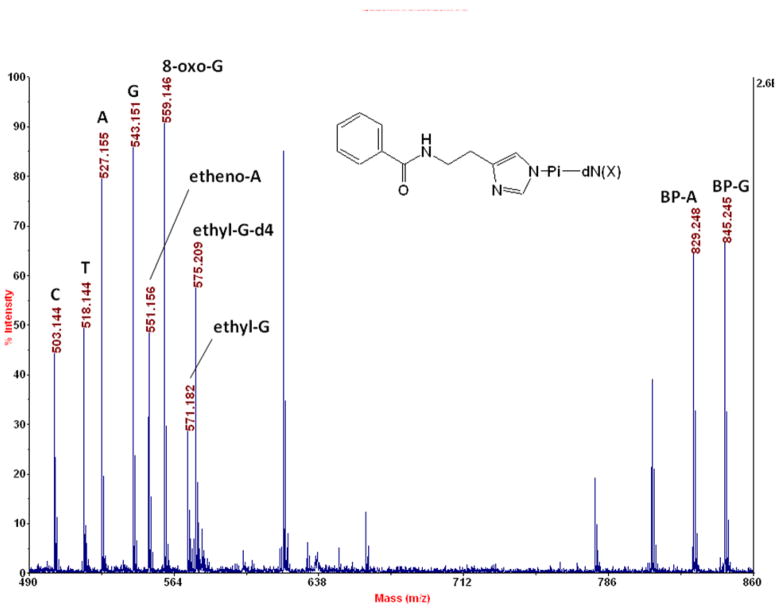
Figure 1.
Detection of four BH-labeled normal deoxynucleotides (C: dCMP at 503.144, T: TMP at 518.144, A: dAMP at 527.155, G: dGMP at 543.151) and five BH-labeled DNA adducts (1,N6-etheno-dAMP at 551.156, 8-oxo-dGMP at 559.146, N2-ethyl-dGMP at 571.182, BP-A at 829.248, and BP-G at 845.245), along with an internal standard (N2-ethyl-d4-dGMP at 575.209) by MALDI-TOF-MS. A sample volume of 0.5 μL containing 50 fmol each of these compounds, that was also 0.5 mg/mL in α-cyano-4-hydroxycinnamic acid (CCA matrix) in 50% in acetonitrile, was deposited manually onto a stainless steel plate (AB SCIEX V70066), and the spectrum was summed from 50 laser pulses on a MALDI-TOF-MS (AB SCIEX Voyager DE STR) in the reflectron mode. BH, benzoylhistamine; dN (the deoxynucleoside part of a normal DNA mononucleotide); dN(X), a DNA adduct; BP-A, benzo[a]pyrene diolepoxide adduct of dAMP; BP-G, benzo[a]pyrene diolepoxide adduct of dGMP. The observed masses matched the exact masses exactly except for A (exact mass 527.156), ethyl-G-d4 (527.207), and BP-A (829.250). Internal calibration was done: the peaks for C, BP-G, and etheno-A were employed, but their masses were allowed to vary in the calculation.