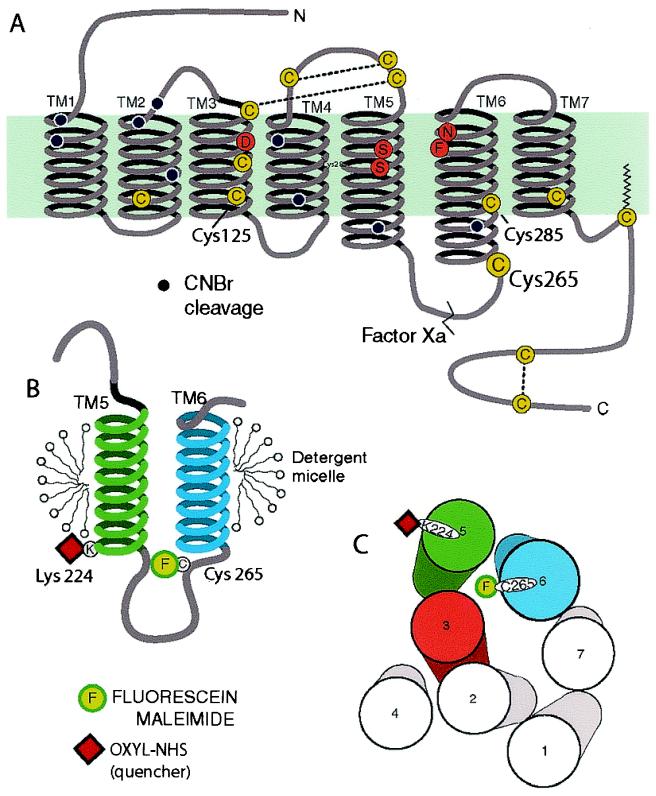
Figure 1.
Schematic diagram of the secondary structure of the β2AR illustrating the FM-labeling site at Cys-265. (A) There are 13 cysteines (yellow circles) in the β2AR, yet only Cys-265 is labeled with the relatively large polar fluorophore FM under the conditions described in Materials and Methods. Cys-106, Cys-184, Cys-190, and Cys-191 have been shown to be disulfide bonded (38, 39), and Cys-341 is palmitoylated (40). Cys-378 and Cys-406 in the carboxyl terminus form a disulfide bond during purification (data not shown). Labeling specificity was confirmed by peptide mapping and mutagenesis of potential reactive cysteines (data not shown). The sites of peptide cleavage by Factor Xa (line) and cyanogen bromide (black dots) are shown. (B) Schematic focusing on TM helices 5 and 6 and the connecting intracellular loop 3 (IC3). The location of the FM (F) site is highlighted. Fluorescence quenchers localized to either the aqueous milieu, the micellar environment, or the base of TM5 (oxyl-NHS bound to Lys-224, red square) were used to monitor conformational changes around Cys-265. (C) Cylinders representing the seven TM helices of the β2AR as viewed from the cytoplasmic side of the membrane, arranged according to the crystal structure of rhodopsin in the inactive state. In the inactive receptor, FM on Cys-265, is predicted to point toward the cytoplasmic extensions of TMs 3, 5, and 6. Also shown is the predicted position of the quencher oxyl-NHS on Lys-224 (red square).