Figure 3.
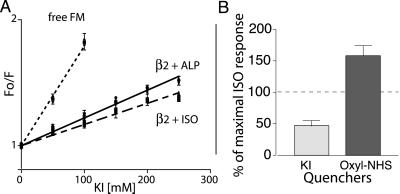
Response of FM-β2R to agonist in the presence of KI or oxyl-NHS. (A) Stern–Volmer plots of KI quenching of FM-labeled β2AR. KI was added to FM reacted with cysteine, to labeled receptor incubated with 20 μM ALP, and to labeled receptor incubated with 100 μM ISO. Fluorescence was measured and plotted as described in Materials and Methods. The quenching constant Ksv was 7.9 ± 0.4 M−1 for fluorescein alone, 2.19 ± 0.06 M−1 for labeled receptor incubated with ALP, and 1.66 ± 0.06 M−1 for labeled receptor incubated with ISO. The difference between ISO and ALP was significant (P < 0.05, unpaired t test). There was no difference in Ksv between buffer alone and ALP treatments. All values are mean ± SEM, n = 3. (B) The effect of quenchers KI and oxyl-NHS on the magnitude of the ISO-induced decrease in fluorescence was determined. Percentage of control ISO response was calculated by using the formula [100 (ISO induced change in fluorescence in the presence of quencher)/(ISO induced change in fluorescence in the absence of quencher)]. For the aqueous quencher KI, the ISO-induced change in fluorescence in the presence of 250 mM KI was less than that in the presence of 250 mM KCl (55.4 ± 8.3% of control ISO response). In contrast, covalent binding of the spin-labeled quencher oxyl-NHS to K224 in TM5 increased the magnitude of the ISO response relative to the control (158 ± 8% control ISO response). In these experiments, the magnitude of the ALP reversal of the ISO-induced change in fluorescence was used as a measure of the magnitude of the ISO response for reasons given in the legend for Fig. 2. All values are mean ± SEM, n = 3.