Abstract
Sertoli cells are immune privileged cells, important for controlling the immune response to male germ cells as well as maintaining the tolerogenic environment in the testis. Additionally, ectopic Sertoli cells have been shown to survive and protect co-grafted cells when transplanted across immunological barriers. The survival of ectopic Sertoli cells has led to the idea that they could be used in cell based gene therapy. In this review, we provide a brief overview of testis immune privilege and Sertoli cell transplantation, factors contributing to Sertoli cell immune privilege, the challenges faced by viral vector gene therapy, the use of immune privileged cells in cell based gene therapy and describe several recent studies on the use of genetically engineered Sertoli cells to provide continuous delivery of therapeutic proteins.
Keywords: cell therapy, gene therapy, immune privilege, Sertoli cells, testis
Introduction
Testis immune privilege is important for preventing a detrimental immune response against the auto-immunogenic germ cells.1 Sertoli cells (SC) are one of the main players responsible for this property of the testis and studies have shown that SC survive long-term when transplanted across immunological barriers, i.e., allo- or xeno-transplantation, without the need of immunosuppressive therapy.2 SC are also capable of prolonging the survival of other cells, such as pancreatic islets, when co-transplanted with the SC.2 More recently, it has been suggested that SC can be used as a vehicle for cell based gene therapy.2 This is supported by studies that showed SC can be genetically engineered to process and secrete biologically active proteins3,4 and that genetically engineered SC retain their immune privilege potential.4,5 These data support a new role for immune privileged SC, which is to use them as a delivery vehicle for therapeutic proteins without tissue rejection or other detrimental host immune responses. This approach, which combines both gene and cell therapy, circumvents problems normally encountered with viral vector gene therapy such as immune-mediated elimination of vector, insertional mutagenesis and low tissue availability. The potential use of SC in cell based gene therapy is vast and could be extended to the treatment of several diseases e.g., hemophilia (factor VIII or IX), type I diabetes (insulin), type II diabetes (glucagon-like peptide-1, GLP-1) and rheumatoid arthritis (antagonists of pro-inflammatory cytokines).
Testis Immune Privilege
The testis is an immune privileged site that not only provides the tolerogenic environment for protection of the maturing, auto-immunogenic germ cells, but also allows for prolonged survival of foreign (allogeneic and xenogeneic) tissues after transplantation into the interstitial space.1,2 The immune privilege status of the testis has been recognized for over two centuries with variable survival of testicular tissue transplants or tissues engrafted into the testis.6 The first study to demonstrate testis immune privilege was performed by John Hunter in 1767 when testes transplanted as allografts into the abdominal cavity of a hen were found to have “perfectly normal structure.”6 Despite this early beginning, it was not until the 1970s and early 1980s that testicular immune privilege was examined more thoroughly.6 During this time, a series of reports documented that various tissues (e.g., skin,7,8 parathyroid fragments,7,9-11 pancreatic islets12,13 and insulinoma tissue14) would survive even when they were grafted into the testes of different species, verifying the testis as an immune privileged site.
In order to identify the mechanism responsible for testis immune privilege, various components were explored including the lower temperature of the scrotum,9,15 impaired lymphatic drainage,9,16 locally produced hormones,17,18 Leydig cells,18 spermatogenesis and germ cells,11,15 and all were found not to be required for testis immune privilege. This suggested that immunoregulatory mechanisms attributed to the remaining cellular components of the testis, primarily SC (also macrophages and possibly peritubular myoid cells), were responsible for creating and maintaining immune privilege in the testis.
Sertoli Cells
SC are somatic cells that together with the germ cells and peritubular myoid cells comprise the seminiferous tubules of the testes. They provide nutrients and factors required for the development and protection of the germ cells.19 Since the germ cells develop after the immune system has determined its set of self antigens that can be tolerated, and possess a profile of novel cell surface markers that can be recognized as foreign by the host immune system,20,21 they should be subjected to immunological rejection. However, SC provide protection to the germ cells by creating a physical barrier (blood-testis barrier/SC barrier) and secreting immunomodulatory factors, thus contributing to testicular immune privilege.
SC immune privilege is attributed to their production of several factors that can control the immune response (reviewed elsewhere).2,22,23 They secrete molecules that inhibit interleukin-2 production and proliferation of B and T lymphocytes.2 They express several complement inhibitors (e.g., decay accelerating factor (DAF), serping1),23 and survive when exposed to an antibody/complement-mediated killing assay.24 Moreover, they express indoleamine-2, 3-dioxygenase (IDO), adhesion molecules (intercellular adhesion molecule 1 (ICAM), vascular cell adhesion molecule 1 (VCAM)), immunoregulatory cytokines (e.g., transforming growth factor β (TGF-β), interleukin-6, activin), chemokines [chemokine (C-C motif) ligand 27 (CCL27)], apoptosis inhibitors (serpina3n, protease inhibitor-9), and enzymes that produce anti-inflammatory prostanoids (prostaglandin E synthase, prostaglandin I synthase).2,22,23 Overall this can lead to immunosuppression and tolerance.
Sertoli Cell Co-transplantation Studies
Small animal models
The importance of SC in creating an immune privileged environment in the testis was confirmed by co-transplantation studies where SC were shown to survive and protect co-transplanted cells in situations (allo- and/or xeno-transplantation) where most other cells were immunologically rejected. The first study to demonstrate that SC survive as allografts and protect other co-transplanted cells was described by Selawry and Cameron in 1993.25 SC were isolated from either PVG or Sprague Dawley rats and transplanted along with islets from Sprague Dawley rats into the renal subcapsular space of diabetic male or female PVG rats. Sixty-five percent of the rats that received SC along with islets and a short course of immunosuppression (cyclosporin) remained normoglycemic for more than 100 d as compared with zero percent of the control group transplanted with islets alone and 30 percent of rats that received islets and treatment with cyclosporin. Modification of the SC isolation procedure and addition of a recovery period (culturing the cells for 48 h) prior to transplantation improved the use of SC such that when transplanted as allografts they could protect islets even without the use of immunosuppressive drugs.26 Since publication of these studies, both the survival of SC as allografts and their ability to protect co-engrafted allogeneic islets has been confirmed by several investigators, as extensively discussed in a recent review.2
The immunoprotective capabilities of SC also include protection of xenogeneic islets when co-transplanted with a short course of immune suppression27,28 or use of encapsulation.29,30 In one study, islets isolated from tilapia fish and SC isolated from Wistar-Furth rats were co-encapsulated and transplanted into diabetic BALB/c mice. The mean graft survival time, as determined by lowering of blood glucose levels, was prolonged significantly to 46 ± 10.9 d in mice transplanted with encapsulated SC and islets as compared with controls that received only encapsulated islets (21 ± 6.7 d).29 Furthermore, the protection of co-transplanted tissue by SC was not limited only to islets. Sanberg et al., showed that SC when co-transplanted with xenogeneic adrenal chromaffin cells into rat brain also protected the transplanted cells, and decreased microglia response at the site of transplantation.31 Similarly, SC have been shown to protect xenogeneic hepatocytes,32 xenogeneic neurons,33 allogeneic34 or xenogeneic skin35 and allogeneic heart grafts.36
Large animal models
Selawry et al., examined the immunoprotective ability of testes in a primate model. They transplanted allogeneic islets into the interstitial space of cryptorchid testes from diabetic Rhesus monkeys37 (also discussed in a review38), receiving a brief course of cyclosporin (20 mg/kg, on days -3 to +3). They found that the animals remained normoglycemic for 8, 54 and 60 mo. After 5 y, removal of the testis from the normoglycemic monkey resulted in a return to hyperglycemia. Tissue obtained for histological examination revealed the presence of insulin-positive islets. Subsequently, porcine islet xenografts were transplanted into the interstitium of dog testes with or without immunosuppression.39 Grafts collected after 100 d were positive for insulin and glucagon staining and no difference in survival of islets was observed between the animals that received immunosuppression vs. those that did not.
Male germ cells isolated from pigs, goats, cattle, dogs, and sheep have also successfully colonized the seminiferous tubules of immune-competent, large animal testes when transplanted as allografts without immune suppression.40-47 In the case of goats and sheep several offspring were produced.43,47 It is important to note that similar experiments performed in rodents resulted in limited colonization unless immune suppression was used.48-50 The reason for the difference in success of these species is unclear.
The immunoprotective role of SC isolated from pigs has also been studied in primates. Neonatal porcine Sertoli cells (NPSC) together with neonatal porcine islets have been transplanted into the omental pouch, kidney, pancreas, and liver of non-diabetic macaques. After 2 mo, tissues were analyzed for cell survival. While inhibin (a marker for SC) and glucagon (a marker for islet α cells) immunoreactive cells were found, no insulin (a marker for islet β cells) positive cells were detected.51
The first clinical trial using SC was performed in Mexico. In this study, initially 12 type I diabetic patients were enrolled and later 11 more patients were added to the group. All the subjects received NPSC co-transplanted with neonatal porcine islets into a device that had been placed into the subcutaneous space of the patients 2 mo prior. After transplantation, exogenous insulin dose requirement was reduced greater than 33% in more than half of the patients52 and half of the patients had improved hemoglobin A1c levels. Two of the patients remained insulin independent for up to 2–3 mo.53 Although porcine insulin was detected at 28 mo in one patient and at 4 y in two patients by HPLC, no porcine C-peptide (an indicator of graft function) was detected in the patient’s blood.53 However, low amounts of porcine C-peptide were detected in the patient’s urine under basal conditions and this value increased after stimulation with l-arginine.52 Additionally, mullerian inhibiting substance (MIS)-positive SC and insulin- and glucagon-positive islets were detected 3 y after transplantation. This trial has faced serious criticism due to safety and ethical concerns associated with clinical transplantation of porcine tissue; and because the procedure was not tested in a large animal model, such as non-human primates, prior to clinical transplantation.54
Survival of Transplanted SC
While the aforementioned studies indicate that SC can protect co-transplanted tissues, the ability of SC to protect themselves is better than the protection they provide to co-transplanted tissues. For example when allogeneic mouse SC were co-transplanted with islets, the survival of co-transplanted islets was successful in ~59% of mice whereas over 90% of the grafts contained numerous SC.55 Additionally, protection of xenogeneic islets requires immune suppression27,28 or encapsulation29,30 while porcine SC can survive without immune suppression.56 Survival of SC as xenografts was first demonstrated by transplanting porcine SC into rat brain.57 The SC survived for 2 mo without immunosuppression. However, the brain is also an immune privileged site in the absence of SC. Later, a study published by Dufour et al., showed that SC isolated from neonatal pigs survived as xenografts for at least 90 d when transplanted into the renal subcapsular space (a non immune privileged site) of non immune suppressed Lewis rats.56 Graft survival was verified by staining with vimentin (NPSC marker) and by PCR for pig specific cytochrome oxidase II. Survival of NPSC for at least 40 d has been confirmed after transplantation to BALB/c mice or female Wistar rats.39,58 NPSC have also survived and protected neonatal pig islets for more than 200 d after transplantation to diabetic C57BL/6 mice (28 Rayat GR, personal communication). However this required a short course of monoclonal antibody therapy. These data suggest that survival of SC is better than the co-transplanted cells and led to the concept that engineered immune privileged SC may be used to deliver various therapeutically relevant proteins.
Gene Therapy
The goal of gene therapy is to replace absent or faulty genes with functional genes and consequently eliminate the root cause of the disease. Hereditary single-gene defects, such as β-thalassemia, were initially considered important candidates for gene therapy. More recently, cancer, cardiovascular disease, diabetes mellitus and neurodegenerative disorders have been added to this list.59
Gene therapy delivery vehicles include non-viral and viral vectors. Non-viral vectors offer advantages such as, being non-pathogenic, less toxic and being produced in relatively large amounts. However, the efficiency of gene transfer is lower than with viral vectors.60 Furthermore, transgene expression tends to be transient and is limited by endosome/lysosomal degradation. Therefore, viral vectors are often the preferred choice for delivering genes of interest because they allow for sustained and high levels of expression of the transgene.60 Viral vectors currently used in gene therapy can be grouped into non-integrating (adenoviral and herpes simplex-1 virus) and integrating [oncoretroviruses, lentiviruses, and adeno-associated viruses (AAV)] categories.59 Although, viral vectors are efficient means of delivering targeted DNA they have also been associated with unwanted and potentially very serious side effects.
The major problem encountered by viral vectors is the host’s immune response. Administration of viral vectors results in generation of innate (mediated by inflammatory cytokines) and adaptive (humoral and cell-mediated) immune responses. Because adenoviral vectors retain most of their viral genome they induce stronger immune responses. The innate immune response is of major concern as it leads to the clearance of approximately 90% of adenoviral vector DNA when injected intravenously.61 The humoral immune response is also a major problem for adenoviral vectors or AAV as humans are a natural host to these viruses and already have preformed neutralizing antibodies and memory B cells against these viruses and their serotypes.61 In addition, a cell-mediated immune response (CD4+ and CD8+ T cells) can also be elicited against adenoviral vector.61 The immunogenicity of adenoviral vectors can be seen in the Gelsinger tragedy that occurred in 1999. In this trial, a second generation adenoviral vector expressing human ornithine transcarbamylase was administered into the hepatic artery of 17 y old Jesse Gelsinger.62 Eighteen hours following gene transfer, the subject started showing signs of systemic inflammatory immune response to the viral vector and later died due to multi-organ system failure.62
In terms of innate immune response, AAV is a weak immunogen and does not elicit a strong inflammatory response.61 However, AAV (mainly AAV2 serotype) has been shown to interact with complement factors, mainly C3, C3b, iC3b thereby increasing its uptake into macrophages and enhancing their activation.61 Recently in a clinical trial, the adaptive immune response to AAV became evident. In this trial, hepatocytes were transduced with rAAV expressing canine Factor IX and infused through the hepatic artery into seven subjects to treat hemophilia. A gradual loss in transgene expression was observed and later studies revealed that the transduced hepatocytes were destroyed by cell-mediated immunity targeting antigens of the AAV capsid.63 Of all the viral vectors described, lentiviral vectors are the least immunogenic. However, it has been shown in vivo that an immune response can be elicited against lentivirus-encoded transgene products.64,65
Besides an immune reaction, another major concern with the use of integrating viral vectors is insertional mutagenesis.59 Although genomic integration is an essential feature to obtain stable expression of the transgene, it is a double-edged sword that can also lead to insertional mutagenesis and potentially cancer if insertion of the vector occurs near proto-oncogenes. This concern became apparent during the clinical gene therapy trials conducted for patients suffering with severe combined immunodeficiency (SCID) using a retroviral vector for transferring the gene of interest.66 Long-term follow up of the clinical trials for SCID-X1 (due to mutation in the gene encoding the common cytokine-receptor γ chain)67 showed evidence of a functional immune system and sustained clinical results in 17 out of 20 patients.68 However, T-cell acute lymphoblastic leukemia developed in five patients.67 Three patients responded to chemotherapy and are in remission but unfortunately one patient died from refractory leukemia.67 The cause of the leukemia-like disorder was due to the insertion of the vector genome near the oncogene, LIM domain only 2.67 This is a cause for concern since insertion of the gamma-retrovirus is not random but more likely to integrate into transcriptionally active regions of the chromatin.69,70 No case of insertional mutagenesis has been reported in ADA-SCID trials [another form of SCID, caused by the lack of adenosine deaminase (ADA) enzyme], but detailed analysis of cells obtained from 5 patients revealed insertion close to proto-oncogenes.67 Besides SCID, retroviral vector gene therapy has been extended to other diseases including Wiskott-Aldrich syndrome71 and chronic granulomatous disease.67 Desirable clinical success has been achieved in Wiskott-Aldrich trial however one of the patients developed T cell leukemia.71 In the chronic granulomatous disease clinical trial, limited initial clinical benefits have been reported in patients due to loss of transgene expression67 and unfortunately, expansion of gene modified cells was also observed in three of the patients (out of 12 subjects who underwent treatment72) due to the insertional activation of growth-promoting genes. One of the patients died of severe sepsis and multiorgan failure 2.5 y after treatment.67
Several in vivo animal studies have shown that while lentiviral vectors can also exhibit insertional mutagenesis, they are generally less mutagenic than gamma-retroviral vectors.73 In the first lentiviral vector clinical trial,74,75 no incidence of abnormal proliferation of cells (leukemia) or other adverse events have been reported, suggesting that lentiviral vectors are safe to be used in gene therapy.74,75 Clinical success comparable to allogeneic hematopoietic stem cell transfer has also been reported in another lentivirus trial with no vector related toxicity or leukemia,76 suggesting that lentiviral vectors could decrease the chance of insertional mutagenesis.
Besides these promising findings, another clinical trial using lentiviral vectors has recently triggered alerts from regulatory bodies.77 In this study, CD34+ hematopoietic stem cells were transduced with a lentiviral vector expressing human β-globin to correct β-thalassemia.78 Despite the promising results, a clonal expansion of erythroid precursors has been reported. Although the exact mechanism of the clonal expansion has to be verified, it has been postulated that it is due to insertion of the vector into the gene locus for high mobility group A2 protein.77 Thus although great advances have been made in order to overcome insertional mutagenesis, no viral vector currently meets desirable clinical safety standards.
Cell-Based Gene Therapy-Utilizing Immune Privileged Cells
Cell based gene therapy is defined as the use of cells carrying a protein of interest to target specific diseases. Cell based gene therapy holds great promise to treat both genetic and acquired diseases. This approach can also overcome viral vector based concern i.e., insertional mutagenesis. For example cells carrying the transgene can be expanded in culture, screened for insertional mutagenesis and the cells expressing high levels of transgene and devoid of vector insertion near proto-oncogenes could be utilized in clinical trials. The major limitation of this approach is that the cells carrying the transgene would be rejected. To escape immune rejection, immune privileged cells [e.g., SC or mesenchymal stem cells (MSC)] could be used in a cell based gene therapy approach.
Using immune privileged cells in cell based gene therapy has been studied by several investigators. We will first discuss the cell based gene therapy approach using MSC. MSC are multipotent stem cells that retain their capacity to differentiate toward chondrogenic, adipogenic and osteoblastic lineages. Recently the immune privileged properties of MSC have been explored more extensively and it has been shown that like SC, they survive the host’s immune response when transplanted across immunological barriers.79-81 Initial experiments performed in 1997, showed efficient MSC transduction with a retroviral vector carrying interleukin-3 gene and laid the foundation of using MSC in cell based gene therapy.82 Later, MSC engineered to produce interleukin-2 or interferon-β, have been shown to reduce tumor growth and prolong animal survival.80 Furthermore, MSC producing Akt1 (pro-survival protein) or human insulin reduce cell death in cardiac ischemia80 and ameliorate diabetes in animal models, respectively.80 Besides the vast potential of MSC as a cell based gene therapy vector their major drawbacks are heterogeneity and tumor formation.80 Recently, two studies utilizing human83 or monkey MSC,84 have highlighted the issue of tumor formation by showing spontaneous transformation of MSC when cultured in vitro. Moreover, when these transformed MSC were injected into NOD/SCID mice subcutaneous tumors developed verifying that they are highly tumorigenic.84 Because differentiated SC do not have the above mentioned problems they are excellent candidates for efficient and safe cell based gene therapy.
Sertoli Cells as a Vehicle for Cell Based Gene Therapy
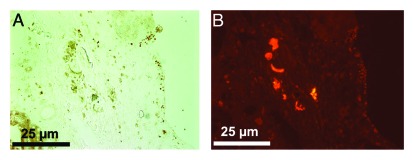
The ability of immune privileged SC to survive after transplantation suggests that they can be engineered to deliver therapeutic proteins. As an initial attempt to explore this possibility, we examined the ability of SC producing green fluorescent protein (GFP) to survive allotransplantation and continue to express GFP.5 SC isolated from transgenic TgN (GFPU) 5Nagy ICR mice engineered to express GFP were transplanted into the renal subcapsular space of BALB/c mice.5 Analysis of the grafts revealed the long-term survival (at least 60 d) of GFP-expressing SC (Fig. 1), with SC detected in 70% of the grafts. When GFP-expressing islets were transplanted as controls, all islet grafts were rejected within 17 d.5 This study mentioned above verified that genetically engineered SC retained their immune privileged status but it did not examine their ability to secrete a clinically relevant, therapeutic protein.5 In 2006, Trivedi et al., modified Lewis rat SC with a recombinant adenoviral vector expressing enhanced GFP (eGFP) and a human trophic factor, neurotrophin-3 (hNT-3), and found that the modified SC secreted biologically active hNT-3 in vitro.4 After transplantation as allografts into the acutely injured spinal cord of Sprague-Dawley rats, the modified SC survived and expressed eGFP for at least 42 d. While significant amounts of hNT-3 were present 3 d post-transplantation, hNT-3 was not detected at later time points.4 Thus, they could not determine whether hNT-3 was beneficial as an in vivo therapy.
Figure 1. SC isolated from transgenic mice (TgN (GFPU) 5Nagy) survive and express GFP when transplanted as allografts in BALB/c mice. Thirteen million SC were transplanted into the renal subcapsular space of BALB/c mice. The graft bearing kidney was collected at day 60 post-transplantation (A and B) and double immunostained for GATA-4 (SC marker, A) and GFP (B).
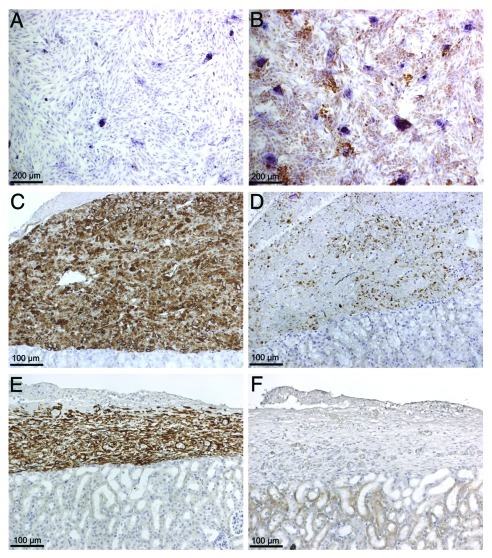
More recently, mouse and porcine SC have been engineered to express basal levels of insulin (nonglucose regulated) to determine whether they are capable of normalizing blood glucose levels in diabetic mice.3 BALB/c mouse and porcine SC transduced with a recombinant adenoviral vector containing furin-modified human insulin cDNA (Ad-CMV-HI), expressed insulin mRNA (data not shown) and protein (Fig. 2B) and secreted significant amounts of insulin into the cell culture media. Moreover, when 20 million cells were transplanted into the renal subcapsular space of diabetic SCID mice there was a significant decrease in blood glucose levels which remained significantly decreased for 5 d.3 However, due to the epichromosomal nature of the adenoviral vector, this decrease in blood glucose level was transient and subsequently returned to the diabetic state within 8 d. The rise in blood glucose correlated with a corresponding loss of insulin expression without evidence of SC death (Fig. 2C–F). Together, these findings suggest that both mouse and porcine SC can be engineered to express a biologically-active and therapeutically relevant protein at levels adequate for the treatment of a disease establishing their utility as novel tools for gene therapy.
Figure 2. Production of insulin protein by NPSC transduced with adenoviral vector carrying furin modified human insulin cDNA. (A and B) NPSC were cultured overnight as a monolayer on chamber slides, transduced with Ad-CMV-HI vector at a MOI of 0 (A) and 100 (B). Slides were collected after 24hrs, fixed with 1% paraformaldehyde and immunostained for insulin (A and B). Nontransduced SC do not express insulin (A), while transduced SC express insulin (brown, B).Twenty million NPSC transduced with Ad-CMV-HI vector (MOI 100) were transplanted into the renal subcapsular space of diabetic SCID mice. Graft bearing kidneys were collected at days 1 (C and D) and 10 (E and F) post-transplantation and immunostained for NPSC marker, vimentin (antibody does not cross react with mouse tissue) (brown, C and E) or insulin (brown, D and F). All the sections were counterstained with hematoxylin (blue).
The adenoviral vector described above allowed for high but transient expression of insulin by SC. In order to produce stable, long-term expression of insulin, a lentiviral vector that contains furin-modified human insulin cDNA upstream of ZsGreen fluorescent protein all under the control of elongation factor 1 α (EF) promoter was created (LVEF-HI-ZsGreen). This vector has been preliminarily tested using a mouse Sertoli cell line (MSC-1). MSC-1 cells are a mouse SC line isolated from C57Bl/6 x SJL transgenic mice that contain the transforming region of the SV40 virus (T antigen) fused to the transcriptional regulatory sequences of human MIS.85 MIS was used in order to direct expression of SV40 to SC. While MSC-1 cells lack many of the immune privileged abilities of primary SC,23 they survive for over 60 d in 88% of recipients when transplanted as allografts into diabetic BALB/c mice.55 Thus, MSC-1 cells are a good model to test the stability and function of the LVEF-HI-ZsGreen lentiviral vector.
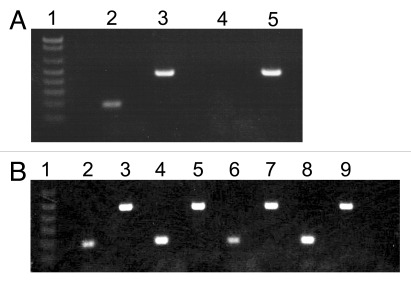
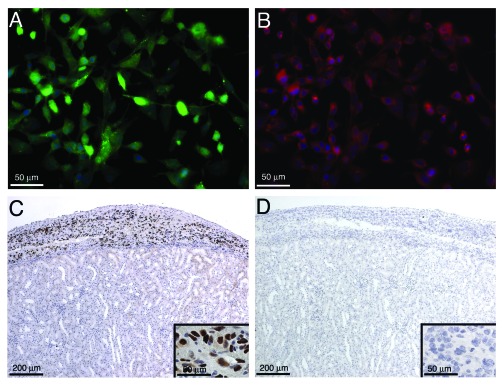
After transduction with the lentiviral vector (LVEF-HI-ZsGreen), MSC-1 cells (MSC-1-LVEF-HI-ZsGreen) expressed insulin mRNA (Fig. 3A) and protein (Fig. 4B) for at least 9 mo indicating stable integration of the insulin construct into the cells. However, the amount of insulin secreted by the cells into the cell culture media was low as determined by ELISA. Twenty million MSC-1-LVEF-HI-ZsGreen cells were transplanted as allografts into the renal subcapsular space of diabetic BALB/c mice. As expected the MSC-1-LVEF-HI-ZsGreen cells were unable to normalize blood glucose levels in the diabetic recipients due to low insulin production and secretion by these cells. Nonetheless, when the grafts were immunostained for cell survival numerous MSC-1-LVEF-HI-ZsGreen cells were detected at 1, 5, 12 and 20 d (Fig. 4C, data shown for day 20). Consistently, insulin mRNA was detected by RT-PCR in grafts collected at days 1, 5, 12 and 20 (Fig. 3B). However, insulin protein was not detected by immunohistochemistry in any of the grafts (Fig. 4D). The lack of insulin immunostaining could be explained by the very low amount of insulin produced by these cells, which could be further masked by tissue embedding and processing.
Figure 3. Insulin mRNA production by MSC-1-LVEF-HI-ZsGreen cells. (A) Transduced MSC-1 cells were grown as monolayer in DMEM plus 10% fetal bovine serum plus 250 ug/ml of G418 for 9 mo. RT-PCR was performed on MSC-1-LV-HI-GFP cells (Lanes 2 and 3) or non-transduced MSC-1 cells (Lanes 4 and 5) for insulin (Lanes 2 and 4) and cyclophilin (Lanes 3 and 5). Lane 1 is the 1kb plus DNA Ladder (Invitrogen). (B) Twenty million transduced MSC-1 cells were transplanted into the renal subcapsular space of diabetic BALB/c mice. RT-PCR was performed on grafts collected at days 1 (Lanes 2 and 3), 5 (Lanes 4 and 5), 12 (Lanes 6 and 7) and 20 (Lanes 8 and 9) post-transplantation for insulin (Lanes 2, 4, 6 and 8) and cyclophilin (Lanes 3, 5, 7 and 9). Lane 1 is the 1kb DNA Ladder (Invitrogen). Care and maintenance of animals described in Figure 3B, 4C and D was performed in accordance with the Institute for Laboratory Animal Research Care and Use of Laboratory Animals, and Texas Tech University Institutional Animal Care and Use Committee-approved protocols.
Figure 4. Immunohistochemical analysis of MSC-1-LVEF-HI-ZsGreen cells and detection of insulin after transplantation. (A and B) MSC-1-LVEF-HI-ZsGreen cells were grown in culture for 9 mo as described in the legend for Figure 3A. Immunofluorescence was performed to detect GFP (A) and insulin (B) protein expression. Cells were counterstained with blue hoechst dye to detect cell nuclei. C-D) Transduced MSC-1 cells (20 million) were transplanted into the renal subcapsular space of diabetic BALB/c mice. The graft bearing kidney was collected at day 20 post-transplantation and immunostained for the MSC-1 cell marker, larger T-antigen (brown, C) and insulin (D). Insets are higher magnification of C and D. Sections were counterstained with hematoxylin (blue).
Although the in vivo lentiviral data lack the ability to show biological function of insulin protein, they provide evidence that genetically engineered SC retained their immune privileged properties, survived long-term, and stably expressed insulin mRNA when transplanted as allografts. Future studies are ongoing to generate a vector which will provide stable, high insulin expression and secretion that will normalize blood glucose levels.
Conclusion and Future Perspectives
In this review, we have summarized the past literature supporting the idea that SC are capable of surviving and protecting co-grafted cells when transplanted across immunological barriers. We have also provided evidence for the potential use of immune privileged SC as a means of delivering therapeutic proteins of interest.
After evaluating the long-term in vivo function of genetically engineered immune privileged SC in rodents, this approach could be extended to generate transgenic mice and pigs expressing proteins of interest specifically by SC which may decrease the problems associated with the use of viral vectors. The advantage of the pig model is that large numbers of transgenic SC could be obtained. Moreover, it will be easy to screen for insertional mutagenesis as only the animals without testicular tumors will be used for transplantation. The limitation of this approach is that it could only be utilized in targeting diseases where cell or tissue specific expression of the transgene is not required.
Besides delivering insulin for type I diabetes, SC could be used to deliver native GLP-1 in diabetic patients, in whom, sulfonylurea, metaformin or both have failed. GLP-1 was shown to enhance pancreatic β-cell mass by stimulating β-cell proliferation and neogenesis in healthy and diabetic rodents.86 Due to the short half-life of GLP-1 (only a few minutes), various GLP-1 analogs with extended half-life have been developed e.g., exenatide, liraglutide or taspoglutide. Recently, clinical trials of taspoglutide were suspended due to hypersensitivity reactions and gastrointestinal side effects.87 Moreover, some of the benefits obtained from native GLP-1 are mediated by GLP-1 metabolites which are not compensated by analogs so more interest has been drawn to native GLP-1 gene therapy.87 Another promising area applicable to SC therapy is rheumatoid arthritis. Approximately, 5 million Americans suffer from this disease and 150,000 new cases are diagnosed each year.88 Although a variety of pro-inflammatory cytokines are involved in causing arthritis, tumor necrosis factor-α and interleukin-1, are the main focus of therapy because besides inducing joint inflammation they also cause bone loss. Systemic or intra-articular delivery of these cytokine’s antagonists, tumor necrosis factor receptor fusion protein or interleukin-1 receptor antagonist protein via injection was proposed as a potential treatment.89 However, rapid loss of these proteins after high dose injections severely limited the long-term efficacy of this treatment and more research has been directed toward delivering these antagonists through gene therapy. Genetically engineered SC may be valuable tools to deliver these antagonists or anti-inflammatory cytokines to ameliorate or delay the onset of severe arthritis, respectively.
To conclude, this review emphasizes the unique immune privileged nature of SC and highlights the potential usefulness of engineered SC as tools for the delivery of biologically active proteins for use in the treatment of a wide variety of clinically relevant diseases. The final frontier involves the development of methods allowing SC to produce bioactive molecules in a physiologically regulated manner. SC are often referred to as “nurse cells” based on their ability to nurture the developing germ cells. It appears calling SC “nurse cells” is fitting given their potential for use in clinical therapy.
Acknowledgments
This work was supported in part by NIH grant HD067400 (to J.MD) from the Eunice Kennedy Shriver NICHD and a Texas Tech University Health Sciences Center Preliminary Data Grant. The authors would like to thank Dr. James C. Hutson (TTUHSC) for critically reading this manuscript.
Glossary
Abbreviations:
- SC
Sertoli cells
- GLP-1
glucagon-like peptide-1
- DAF
decay accelerating factor
- IDO
indoleamine-2, 3-dioxygenase
- ICAM
intercellular adhesion molecule 1
- VCAM
vascular cell adhesion molecule 1
- TGFβ
transforming growth factor beta
- NPSC
neonatal pig Sertoli cells
- MIS
Mullerian inhibiting substance
- AAV
adeno-associated virus
- SCID
severe combined immunodeficiency
- ADA
adenosine deaminase enzyme
- GFP
green fluorescent protein
- MSC
mesenchymal stem cells
- NOD
non-obese diabetic
- eGFP
enhanced green fluorescent protein
- hNT-3
human neurotrophin-3
- EF
elongation factor 1α
- MSC-1
mouse Sertoli cell line-1
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/19119
References
- 1.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. doi: 10.1016/S0065-2776(08)60930-X. [DOI] [PubMed] [Google Scholar]
- 2.Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- 3.Halley K, Dyson EL, Kaur G, Mital P, Uong PM, Dass B, et al. Delivery of a therapeutic protein by immune-privileged Sertoli cells. Cell Transplant. 2010;19:1645–57. doi: 10.3727/096368910X516628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi AA, Igarashi T, Compagnone N, Fan X, Hsu JY, Hall DE, et al. Suitability of allogeneic sertoli cells for ex vivo gene delivery in the injured spinal cord. Exp Neurol. 2006;198:88–100. doi: 10.1016/j.expneurol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JM, Hemendinger R, Halberstadt CR, Gores P, Emerich DF, Korbutt GS, et al. Genetically engineered Sertoli cells are able to survive allogeneic transplantation. Gene Ther. 2004;11:694–700. doi: 10.1038/sj.gt.3302218. [DOI] [PubMed] [Google Scholar]
- 6.Maddocks S, Setchell BP. Recent evidence for immune privilege in the testis. J Reprod Immunol. 1990;18:9–18. doi: 10.1016/0165-0378(90)90021-W. [DOI] [PubMed] [Google Scholar]
- 7.Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983;36:423–31. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Whitmore WF, Gittes RF. Studies on the prostate and testis as immunologically privileged sites. Cancer Treat Rep. 1977;61:217–22. [PubMed] [Google Scholar]
- 9.Head JR, Billingham RE. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation. 1985;40:269–75. doi: 10.1097/00007890-198509000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Naji A, Barker CF. The influence of histocompatibility and transplant site on parathyroid allograft survival. J Surg Res. 1976;20:261–7. doi: 10.1016/0022-4804(76)90012-3. [DOI] [PubMed] [Google Scholar]
- 11.Whitmore WF, 3rd, Karsh L, Gittes RF. The role of germinal epithelium and spermatogenesis in the privileged survival of intratesticular grafts. J Urol. 1985;134:782–6. doi: 10.1016/s0022-5347(17)47438-6. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson J, Scothorne RJ. Extended survival of pancreatic islet allografts in the testis of guinea-pigs. J Anat. 1977;124:1–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson J, Scothorne RJ. Further studies on the transplantation of isolated pancreatic islets. J Anat. 1977;124:9–20. [PMC free article] [PubMed] [Google Scholar]
- 14.Akimaru K, Stuhlmiller GM, Seigler HF. Allotransplantation of insulinoma into the testis of diabetic rats. Transplantation. 1981;32:227–32. doi: 10.1097/00007890-198109000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Selawry HP, Whittington K. Extended allograft survival of islets grafted into intra-abdominally placed testis. Diabetes. 1984;33:405–6. doi: 10.2337/diabetes.33.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Head JR, Neaves WB, Billingham RE. Reconsideration of the lymphatic drainage of the rat testis. Transplantation. 1983;35:91–5. doi: 10.1097/00007890-198301000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Selawry HP, Whittington KB. Prolonged intratesticular islet allograft survival is not dependent on local steroidogenesis. Horm Metab Res. 1988;20:562–5. doi: 10.1055/s-2007-1010885. [DOI] [PubMed] [Google Scholar]
- 18.Cameron DF, Whittington K, Schultz RE, Selawry HP. Successful islet/abdominal testis transplantation does not require Leydig cells. Transplantation. 1990;50:649–53. doi: 10.1097/00007890-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–6. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 20.O’Rand MG, Romrell LJ. Appearance of cell surface auto- and isoantigens during spermatogenesis in the rabbit. Dev Biol. 1977;55:347–58. doi: 10.1016/0012-1606(77)90178-6. [DOI] [PubMed] [Google Scholar]
- 21.Tung PS, Fritz IB. Specific surface antigens on rat pachytene spermatocytes and successive classes of germinal cells. Dev Biol. 1978;64:297–315. doi: 10.1016/0012-1606(78)90080-5. [DOI] [PubMed] [Google Scholar]
- 22.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–8. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, et al. Immunoprotective Properties of Primary Sertoli Cells in Mice: Potential Functional Pathways That Confer Immune Privilege. Biol Reprod. 2012;86:1–14. doi: 10.1095/biolreprod.110.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufour JM, Hamilton M, Rajotte RV, Korbutt GS. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol Reprod. 2005;72:1224–31. doi: 10.1095/biolreprod.104.038315. [DOI] [PubMed] [Google Scholar]
- 25.Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–9. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 26.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–22. doi: 10.2337/diabetes.46.2.317. [DOI] [PubMed] [Google Scholar]
- 27.Dufour JM, Rajotte RV, Kin T, Korbutt GS. Immunoprotection of rat islet xenografts by cotransplantation with sertoli cells and a single injection of antilymphocyte serum. Transplantation. 2003;75:1594–6. doi: 10.1097/01.TP.0000058748.00707.88. [DOI] [PubMed] [Google Scholar]
- 28.Ramji QA, Bayrack K, Arefanian H, Marcet-Palacios M, Bleackley RC, Rajotte RV, et al. Protection of porcine islet xenografts in mice using sertoli cells and monoclonal antibodies. Transplantation. 2011;92:1309–15. doi: 10.1097/TP.0b013e3182384ab0. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Wright JR., Jr. Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation. 1999;67:815–20. doi: 10.1097/00007890-199903270-00006. [DOI] [PubMed] [Google Scholar]
- 30.Luca G, Calafiore R, Basta G, Ricci M, Calvitti M, Neri L, et al. Improved function of rat islets upon co-microencapsulation with Sertoli’s cells in alginate/poly-L-ornithine. AAPS PharmSciTech. 2001;2:E15. doi: 10.1208/pt020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol. 1996;14:1692–5. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- 32.Rahman TM, Diakanov I, Selden C, Hodgson H. Co-transplantation of encapsulated HepG2 and rat Sertoli cells improves outcome in a thioacetamide induced rat model of acute hepatic failure. Transpl Int. 2005;18:1001–9. doi: 10.1111/j.1432-2277.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 33.Willing AE, Othberg AI, Saporta S, Anton A, Sinibaldi S, Poulos SG, et al. Sertoli cells enhance the survival of co-transplanted dopamine neurons. Brain Res. 1999;822:246–50. doi: 10.1016/S0006-8993(99)01128-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee HM, Lim HG, Oh BC, Park CS, Lee DS, Lee JR. Systemic immune modulation using chemokine receptor 7 expressing porcine Sertoli cells. Xenotransplantation. 2007;14:619–26. doi: 10.1111/j.1399-3089.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- 35.Shamekh R, El-Badri NS, Saporta S, Pascual C, Sanberg PR, Cameron DF. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006;15:45–53. doi: 10.3727/000000006783982205. [DOI] [PubMed] [Google Scholar]
- 36.Lim HG, Lee HM, Oh BC, Lee JR. Cell-mediated immunomodulation of chemokine receptor 7-expressing porcine sertoli cells in murine heterotopic heart transplantation. J Heart Lung Transplant. 2009;28:72–8. doi: 10.1016/j.healun.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Selawry HP. Islet transplantation to immuneprivileged sites. In: Chick RLaW, ed. Austin, TX: Landes/CRC Press, 1994. [Google Scholar]
- 38.Dufour JM, Rajotte RV, Korbutt GS, Emerich DF. Harnessing the immunomodulatory properties of Sertoli cells to enable xenotransplantation in type I diabetes. Immunol Invest. 2003;32:275–97. doi: 10.1081/IMM-120025106. [DOI] [PubMed] [Google Scholar]
- 39.Gores PF, Hayes DH, Copeland MJ, Korbutt GS, Halberstadt C, Kirkpatrick SA, et al. Long-term survival of intratesticular porcine islets in nonimmunosuppressed beagles. Transplantation. 2003;75:613–8. doi: 10.1097/01.TP.0000052376.89400.8D. [DOI] [PubMed] [Google Scholar]
- 40.Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–8. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- 41.Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41:124–8. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 42.Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64:422–8. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 43.Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–4. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 44.Herrid M, Vignarajan S, Davey R, Dobrinski I, Hill JR. Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction. 2006;132:617–24. doi: 10.1530/rep.1.01125. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–31. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Sosa JR, Silvertown JD, Foster RA, Medin JA, Hahnel A. Transduction and transplantation of spermatogonia into the testis of ram lambs through the extra-testicular rete. Reprod Domest Anim. 2009;44:612–20. doi: 10.1111/j.1439-0531.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 47.Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905. doi: 10.1095/biolreprod.109.078279. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–72. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- 49.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Honjo T, et al. Allogeneic offspring produced by male germ line stem cell transplantation into infertile mouse testis. Biol Reprod. 2003;68:167–73. doi: 10.1095/biolreprod.102.008516. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Renfree MB, Short RV. Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod. 2003;68:961–7. doi: 10.1095/biolreprod.102.009480. [DOI] [PubMed] [Google Scholar]
- 51.Wang DZ, Skinner S, Elliot R, Escobar L, Salto-Tellez M, Garkavenko O, et al. Xenotransplantation of neonatal porcine islets and Sertoli cells into nonimmunosuppressed streptozotocin-induced diabetic rats. Transplant Proc. 2005;37:470–1. doi: 10.1016/j.transproceed.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 52.Valdes-Gonzalez R, Rodriguez-Ventura AL, White DJ, Bracho-Blanchet E, Castillo A, Ramírez-González B, et al. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010;162:537–42. doi: 10.1111/j.1365-2249.2010.04273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419–27. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 54.Birmingham K. Skepticism surrounds diabetes xenograft experiment. Nat Med. 2002;8:1047. doi: 10.1038/nm1002-1047. [DOI] [PubMed] [Google Scholar]
- 55.Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 2008;17:525–34. doi: 10.3727/096368908785096033. [DOI] [PubMed] [Google Scholar]
- 56.Dufour JM, Rajotte RV, Seeberger K, Kin T, Korbutt GS. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation. 2003;10:577–86. doi: 10.1034/j.1399-3089.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 57.Saporta S, Cameron DF, Borlongan CV, Sanberg PR. Survival of rat and porcine Sertoli cell transplants in the rat striatum without cyclosporine-A immunosuppression. Exp Neurol. 1997;146:299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- 58.Yin Z, Chen D, Hu F, Ruan Y, Li J, Wang L, et al. Cotransplantation with xenogenetic neonatal porcine sertoli cells significantly prolongs islet allograft survival in nonimmunosuppressive rats. Transplantation. 2009;88:339–45. doi: 10.1097/TP.0b013e3181ae5dcf. [DOI] [PubMed] [Google Scholar]
- 59.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 60.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–9. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 61.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 64.Kremer KL, Dunning KR, Parsons DW, Anson DS. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J Gene Med. 2007;9:362–8. doi: 10.1002/jgm.1025. [DOI] [PubMed] [Google Scholar]
- 65.Limberis MP, Bell CL, Heath J, Wilson JM. Activation of transgene-specific T cells following lentivirus-mediated gene delivery to mouse lung. Mol Ther. 2010;18:143–50. doi: 10.1038/mt.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 67.Qasim W, Gaspar HB, Thrasher AJ. Progress and prospects: gene therapy for inherited immunodeficiencies. Gene Ther. 2009;16:1285–91. doi: 10.1038/gt.2009.127. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser J. Clinical research. Gene therapists celebrate a decade of progress. Science. 2011;334:29–30. doi: 10.1126/science.334.6052.29. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–51. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 70.Lewinski MK, Yamashita M, Emerman M, Ciuffi A, Marshall H, Crawford G, et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS Pathog. 2006;2:e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galy A, Thrasher AJ. Gene therapy for the Wiskott-Aldrich syndrome. Curr Opin Allergy Clin Immunol. 2011;11:545–50. doi: 10.1097/ACI.0b013e32834c230c. [DOI] [PubMed] [Google Scholar]
- 72.Grez M, Reichenbach J, Schwäble J, Seger R, Dinauer MC, Thrasher AJ. Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther. 2011;19:28–35. doi: 10.1038/mt.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 74.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103:17372–7. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang GP, Levine BL, Binder GK, Berry CC, Malani N, McGarrity G, et al. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol Ther. 2009;17:844–50. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 77.Williams DA. Gene therapy continues to mature and to face challenges. Mol Ther. 2009;17:1305–6. doi: 10.1038/mt.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaiser J. Gene therapy. Beta-thalassemia treatment succeeds, with a caveat. Science. 2009;326:1468–9. doi: 10.1126/science.326.5959.1468-b. [DOI] [PubMed] [Google Scholar]
- 79.Dai LJ, Moniri MR, Zeng ZR, Zhou JX, Rayat J, Warnock GL. Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 2011;305:8–20. doi: 10.1016/j.canlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Aquino JB, Bolontrade MF, García MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17:692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 81.Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62:1156–66. doi: 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allay JA, Dennis JE, Haynesworth SE, Majumdar MK, Clapp DW, Shultz LD, et al. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997;8:1417–27. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
- 83.Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 84.Ren Z, Wang J, Zhu W, Guan Y, Zou C, Chen Z, et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res. 2011;317:2950–7. doi: 10.1016/j.yexcr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster RL, et al. Directed expression of an oncogene to Sertoli cells in transgenic mice using mullerian inhibiting substance regulatory sequences. Mol Endocrinol. 1992;6:1403–11. doi: 10.1210/me.6.9.1403. [DOI] [PubMed] [Google Scholar]
- 86.Combettes MM. GLP-1 and type 2 diabetes: physiology and new clinical advances. Curr Opin Pharmacol. 2006;6:598–605. doi: 10.1016/j.coph.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Riedel MJ, Kieffer TJ. Treatment of diabetes with glucagon-like peptide-1 gene therapy. Expert Opin Biol Ther. 2010;10:1681–92. doi: 10.1517/14712598.2010.532786. [DOI] [PubMed] [Google Scholar]
- 88.Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–80. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 89.Kofron MD, Laurencin CT. Orthopaedic applications of gene therapy. Curr Gene Ther. 2005;5:37–61. doi: 10.2174/1566523052997488. [DOI] [PubMed] [Google Scholar]