Abstract
Objective
To evaluate the effectiveness of GnRH antagonist multiple dose protocol applied during early and late follicular phase (MDP-EL) in comparison with standard GnRH agonist luteal long protocol (LP) in each non-obese and obese polycystic ovary syndrome (PCOS) women undergoing IVF.
Methods
Two hundred eleven infertile women with PCOS were recruited and randomized to undergo either GnRH antagonist MDP-EL (antagonist group) or standard GnRH agonist luteal LP (agonist group). IVF cycle outcomes were compared between the two groups.
Results
Total dose and days of recombinant human follicle stimulating hormone (rhFSH) administered were significantly fewer in the antagonist group than in the agonist group. Incidence of severe ovarian hyperstimulation syndrome was significantly lower in the antagonist group. However, IVF and pregnancy outcomes were similar in the two groups. When all subjects were divided into non-obese and obese subgroups, in non-obese PCOS subgroup, IVF and pregnancy outcomes were comparable in the antagonist and agonist groups but total dose and days of rhFSH were also significantly fewer in the antagonist group. Similar findings were also observed in obese PCOS subgroup.
Conclusion
GnRH antagonist MDP-EL is at least as effective as GnRH agonist LP and may be a more patient-friendly alternative in controlled ovarian stimulation for PCOS patients undergoing IVF, independent of body mass index.
Keywords: Polycystic ovary syndrome, GnRH antagonist, GnRH agonist, Body mass index, Controlled ovarian stimulation, In vitro fertilization
Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility, affecting 5% to 10% of women of reproductive age [1]. PCOS is associated with elevated androgen levels and/or chronic anovulation, ultimately leading to menstrual disorders including amenorrhea, oligomenorrhea, and infertility [2].
Infertile patients with PCOS women are frequently faced with difficult problems in ovulation induction (OI) or controlled ovarian stimulation (COS). Hypersecretion of LH and hyperandrogenemia are thought to be associated with decreased oocyte quality, lower fertilization, lower implantation lower pregnancy, and increased miscarriage rates [3]. Therefore, there is a theoretical benefit in the use of GnRH analogues in COS cycles, especially in patients with PCOS. Actually, GnRH agonists administration before and during ovarian stimulation causes a significant reduction in the incidence of premature LH surges and the frequency of cycle cancellation, thus increasing the success rate of IVF-ET [4,5]. Therefore, GnRH agonist long protocol (LP) has been commonly used for IVF cycles in PCOS patients. However, GnRH agonist LP has certain drawbacks, including the need to administer the agents for more than 2 weeks to induce pituitary desensitization, an unnecessary elevation of gonadotropin levels during early administration of GnRH agonists, and high costs attributable to the increased total dose and a higher number of days of gonadotropin administration. Moreover, this protocol cannot reduce the incidence of ovarian hyperstimulation syndrome (OHSS) in COS cycles. This shortcoming of GnRH agonist LP may be more problematic in PCOS patients whose ovaries are very sensitive to stimulation and often develop excessive follicular development in response to exogeneous gonadotropin.
GnRH antagonists that were recently introduced for COS have several advantages over GnRH agonists. They bind competitively to the GnRH receptors in pituitary, and produce an immediate and rapid decrease in LH and FSH levels without GnRH receptor desensitization as well as flare-up effect. COS protocols using GnRH antagonists have been shown to be effective in preventing premature LH surge [6,7]. Furthermore, meta-analyses in general population demonstrated that GnRH antagonist protocols reduce the incidence of OHSS as well as the amount of gonadotropins used and the duration of stimulation as compared with GnRH agonist protocols [8,9]. However, these meta-analyses showed an inconsistent results on pregnancy outcome. Moreover, there are very limited studies comparing GnRH antagonist protocol and GnRH agonist LP in PCOS patients [10-14] and the optimal stimulation protocol for these patients is still under debate.
Therefore, this study was performed to investigate the effectiveness of GnRH antagonist multiple dose protocol applied during early and late follicular phase (MDP-EL) compared with the standard GnRH agonist luteal LP in PCOS patients undergoing IVF-ET.
Methods
1. Patient population
Our prospective randomized study was performed at a university-based infertility clinic at the Asan Medical Center, Seoul, South Korea. The study population consisted of 211 infertile women with PCOS who had undergone 211 IVF cycles between January 2003 and March 2008.
The Institutional Review Board of our center approved the study and all patients provided written informed consent. The subjects, aged 25 to 39 years, were randomized into either the GnRH antagonist MDP-EL (antagonist group, n=106) or the GnRH agonist LP (agonist group, n=105) by the use of sealed envelopes and a computer-generated list. The sequence of allocation to the two groups was provided to the investigating physicians and randomization was performed as planned according to the randomization list order.
The PCOS diagnosis was based on the revised PCOS diagnostic criteria of the 2003 Rotterdam consensus [15]. All patients were in good health with normal cardiac, hepatic and renal functions, and they had experienced spontaneous onset of puberty and normal sexual development. None of subjects had ever taken any hormonal therapy within the preceding three months.
2. Ovarian stimulation protocols
All patients received oral contraceptive (OC, Diane35; Bayer Schering Pharma, Berlin, Germany) pretreatment for 21 days in the cycle preceding ovarian stimulation. In the antagonist group, 5 days after discontinuation of OC, ovarian stimulation was commenced using 50 to 150 IU of recombinant human FSH (rhFSH, Gonal-F; Merck Serono SA, Geneva, Switzerland) after establishing ovarian and uterine quiescence using vaginal ultrasound. RhFSH was administered in a step-up fashion and the dose of rhFSH was adjusted every 3 to 4 days according to ovarian response. GnRH antagonist, cetrorelix (Cetrotide; Merck Serono SA) 0.125 mg/day was administered in the morning of stimulation day 1 and 2. When the mean diameter of lead follicle reached 13 mm, cetrorelix at a dose of 0.25 mg/day was started again and continued daily up to the day of recombinant hCG (rhCG, Ovidrel; Merck Serono SA) injection.
In the agonist group, GnRH agonist, triptorelin (Decapeptyl; Ferring, Malmo, Sweden) at a dose of 0.1 mg/day was initiated from day 18 of OC pretreatment cycle. All patients had withdrawal bleeding after discontinuation of OC. When pituitary desensitization was achieved, ovarian stimulation was started and the dose of triptorelin was reduced to 0.05 mg daily and continues up to day of rhCG administration. Ovarian stimulation was performed in the same manner with the antagonist group.
In both antagonist and agonist groups, rhCG of 250 µg was administered subcutaneously to induce follicular maturation when one or more follicles reached a mean diameter of 17 mm. Transvaginal ultrasound-guided oocyte retrieval was performed 36 hours after rhCG injection, and one to four embryos, after IVF or ICSI, were transferred into the uterus on the 3rd day after oocyte retrieval. Luteal support was provided by administering 90 mg of vaginal progesterone gel (Crinone gel 8%; Merck Serono SA) once daily from the day of oocyte retrieval. Pregnancies were confirmed by rising serum β-hCG concentrations and transvaginal ultrasonographic evidence of a gestational sac. The serum level of β-hCG was measured 11 days after embryo transfer (ET). Measurement of β-hCG was performed by radioimmunoassay using a hCG MAIAclone kit (Serono Diagnostics, Woking, Surrey, UK). Inter- and intra-assay variances were not more than 10% and 5%, respectively. Measurement of estradiol and progesterone was performed by radioimmunoassay using a Coat-A-Count Estradiol kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) and a Coat-A-Count Progesterone kit (Siemens Medical Solutions Diagnostics); interassay and intraassay variances in measuring estradiol and progesterone were less than 10% and 5%, respectively.
3. Outcome measures
Primary efficacy endpoint was clinical pregnancy rate per cycle. Secondary efficacy variables included total amount and days of rhFSH administered, the numbers of retrieved, mature, fertilized oocytes and good quality embryos, embryo implantation rate, miscarriage rate, and the incidence of severe OHSS. OHSS was diagnosed and classified in accordance with the classification system proposed by Golan et al. [16]. Clinical pregnancy was defined as the presence of a gestational sac by ultrasonography, while miscarriage rate per clinical pregnancy was defined as the proportion of patients who failed to continue development before 24 weeks of gestation in all clinical pregnancies. Preterm birth was defined as the delivery at or before 34 weeks of gestation. Live birth was defined as the delivery of a fetus with signs of life after twenty completed weeks of gestational age.
4. Statistical analysis
The mean value was expressed as the mean±SD. Statistical analysis for the comparisons of mean values was performed using Student's t-test. Chi-square test and Fisher's exact test were used for the comparisons of fraction. Statistical significance was defined as p<0.05. All analyses were performed using the SPSS ver. 11.0 (SPSS Inc, Chicago, IL, USA).
Results
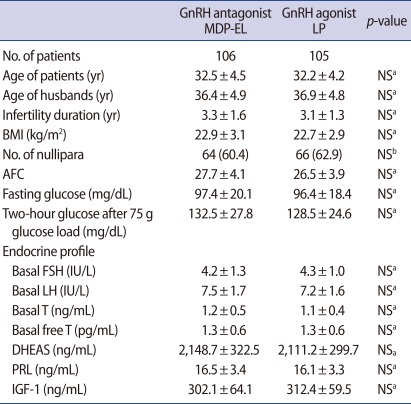
The antagonist group and agonist group were comparable with respect to patients' characteristics such as the age of patients, infertility duration, body mass index (BMI), the proportion of nullipara, antral follicle count, basal endocrine profile and 2-hour glucose level after a 75 g glucose load (Table 1).
Table 1.
Patients' characteristics
Values are presented as mean±SD or number (%).
MDP-EL, multiple-dose protocol during early and late follicular phase; LP, long protocol; NS, not significant; BMI, body mass index; AFC, antral follicle count; IGF-1, Insulin like growth factor-1.
aStudent's t-test; bChi-square test.
The antagonist group consisted of 106 cycles initiated corresponding to 106 patients, and the agonist group consisted of 105 cycles initiated corresponding to 105 patients. One cycle (0.9%) in the antagonist group and 2 cycles (1.9%) in the agonist group were cancelled after oocyte retrieval due to a high risk of OHSS. There was no significant difference in cycle cancellation rate between the two groups.
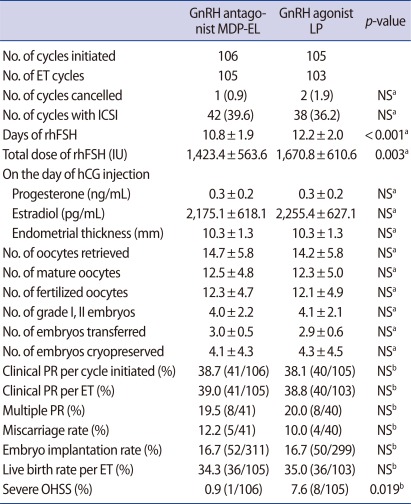
Table 2 presents the comparison of COS results and IVF outcomes between the antagonist and agonist groups. Total days and dose of rhFSH required for COS were significantly fewer in the antagonist group than in the agonist group (p<0.001 and p=0.003, respectively) (Table 2). There were no significant differences between the two groups with respect to serum concentrations of progesterone and estradiol on the day of hCG injection, and the numbers of oocytes retrieved, mature oocytes, fertilized oocytes, grade I or II embryos, embryos transferred and embryos cryopreserved (Table 2). The two groups were similar in the clinical pregnancy rate per cycle initiated and per ET cycle, embryo implantation rate, live birth rate per ET cycle and multiple pregnancy rate (Table 2). However, the incidence of severe OHSS was significantly lower in the antagonist group of 0.9% (1/106), compared with 7.6% (8/105) in the agonist group (p=0.019) (Table 2). Of all patients with severe OHSS, 1 in the antagonist group and 7 in the agonist group became pregnant. All of the patients who suffered from severe OHSS were hospitalized and discharged without any complications.
Table 2.
Comparison of COS results and IVF/ICSI outcome in all subjects
Values are presented as number (%), mean±SD, or % (number/total number).
COS, controlled ovarian stimulation; MDP-EL, multiple-dose protocol during early and late follicular phase; LP, long protocol; ET, embryo transfer; NS, not significant; rhFSH, recombinant human follicle stimulating hormone; PR, pregnancy rate; OHSS, ovarian hyperstimulation syndrome.
aStudent's t-test; bChi-square test or Fisher's exact test.
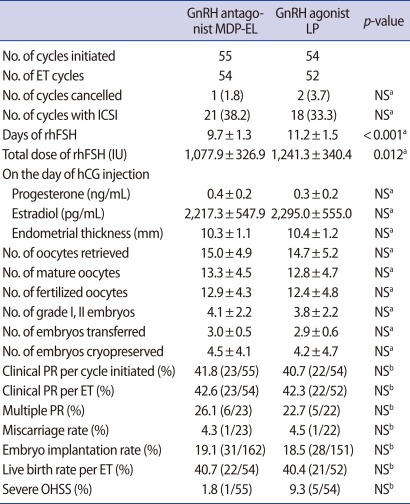
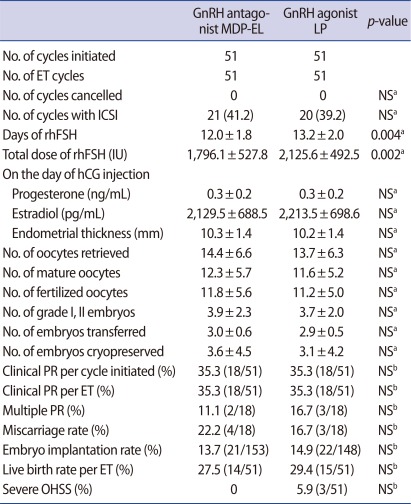
All subjects were divided into non-obese subgroup (BMI<23 kg/m2) and obese subgroup (BMI≥23 kg/m2). In the non-obese subgroup, total days and dose of rhFSH required were also significantly fewer in the antagonist group than in the agonist group (p<0.001 and p=0.012, respectively) (Table 3). IVF and pregnancy outcomes were also comparable in the two groups (Table 3). The incidence of severe OHSS seemed to be lower in the antagonist group, but the difference did not achieve a statistical significance (p=0.113) (Table 3). Similar results were observed in the obese subgroup consisted of PCOS women with BMI≥23 kg/m2 (Table 4).
Table 3.
Comparison of COS results and IVF/ICSI outcome in patients with BMI<23 kg/m2
Values are presented as number (%), mean±SD, or % (number/total number).
COS, controlled ovarian stimulation; MDP-EL, multiple-dose protocol during early and late follicular phase; LP, long protocol; ET, embryo transfer; NS, not significant; rhFSH, recombinant human follicle stimulating hormone; PR, pregnancy rate; OHSS, ovarian hyperstimulation syndrome.
aStudent's t-test; bChi-square test or Fisher's exact test.
Table 4.
Comparison of COS results and IVF/ICSI outcomes in patients with BMI≥23 kg/m2
Values are presented as number (%), mean±SD, or % (number/total number).
COS, controlled ovarian stimulation; MDP-EL, multiple-dose protocol during early and late follicular phase; LP, long protocol; ET, embryo transfer; NS, not significant; rhFSH, recombinant human follicle stimulating hormone; PR, pregnancy rate; OHSS, ovarian hyperstimulation syndrome.
aStudent's t-test; bChi-square test or Fisher's exact test.
Discussion
This study evaluated the effectiveness of GnRH antagonist MDP-EL compared with the standard GnRH agonist luteal LP in PCOS patients undergoing IVF-ET. We used the GnRH antagonist MDP-EL to correct for hormonal abnormalities seen during the early follicular phase, rather than employing the standard GnRH antagonist MDP during the late follicular phase. Our findings suggest that the GnRH antagonist protocol for COS can be applied to PCOS patients and GnRH antagonist MDP-EL may be beneficial in reducing total days and dose of rhFSH required and the incidence of severe OHSS in PCOS patients undergoing IVF-ET.
Particular attention should be paid during OI or COS in PCOS patients, because ovaries of PCOS patients have a surplus number of small antral follicles and these patients are exposed to the risks of OHSS. Despite special attention, complications, such as OHSS, multiple pregnancy and premature luteinization, are usually more frequent in women with PCOS [17]. Other characteristics of women with PCOS, including elevated basal serum LH, hyperandrogenemia, and increased intrafollicular androgen levels have been shown to adversely affect COS or IVF results by interrupting the development of normal follicles and inducing degeneration of mature follicles. The miscarriage rate is therefore higher in PCOS than in normal women [3]. To overcome these problems, GnRH agonist was introduced for COS in PCOS patients and its use effectively prevented premature luteinization, increased pregnancy rate, and decreased miscarriage frequency [4,5]. However, the use of GnRH agonist cannot reduce the incidence of OHSS in PCOS patients undergoing COS. This is an important reason to seek new treatment option than GnRH agonist LP in PCOS patients at high risk of OHSS.
Recently, GnRH antagonist protocols have been reported to reduce the incidence of OHSS as well as days and dose of gonadotropins required when compared with GnRH agonist protocols [8,9] and therefore the use of GnRH antagonists has been proposed as a new safer stimulation option for PCOS patients. However, there is limited number of published studies on the use of GnRH antagonists in COS for PCOS patients, and an optimal GnRH antagonist protocol for these patients is still controversial.
During COS using GnRH antagonists in PCOS women, elevated LH and estradiol levels in the early follicular phase may adversely affect oocyte or embryo quality and may decrease endometrial acceptability and pregnancy rates [15]. For this reason, it has been recommended to use GnRH antagonists in the early follicular phase of GnRH antagonist cycles in these patients [11-13]. Elkind-Hirsch et al. [11] demonstrated the effectiveness and safety of concurrent ganirelix and follitropin-beta therapy for COS in women with PCOS. In addition, pregnancy outcomes in PCOS patients pretreated with OC followed by concomitant administration of cetrorelix from the first day of stimulation, were similar to those of PCOS patients treated with GnRH agonist LP [12]. In our preliminary study [13], two additional use of GnRH antagonist in the early follicular phase of standard GnRH antagonist MDP cycle also yielded comparable IVF results and pregnancy outcome with fewer dose and days of rhFSH used when compared with those of GnRH agonist LP. The present study corroborates the findings of our preliminary study. Meta-analysis by Griesinger et al. [18] showed similar pregnancy outcomes when GnRH antagonist protocols and GnRH agonist LP were compared in PCOS patients undergoing IVF-ET. In a randomized controlled trial (RCT) by Lainas et al. [14], even standard GnRH antagonist MDP also resulted in a similar pregnancy rate and lower incidence of severe OHSS, compared with GnRH agonist LP. So far, there are limited data on COS using GnRH antagonist in PCOS patients and furthermore, there are very few published studies supporting benefits of GnRH antagonist protocols in PCOS patients.
This study showed that GnRH antagonist protocol reduces the incidence of severe OHSS in PCOS patients as compared with GnRH agonist LP. In RCT by Lainas et al. [14], severe OHSS also occurred less frequently in the antagonist group than in the agonist group, although same dose of hCG was administered in both groups. The explanation for this finding is that GnRH antagonist itself may have a slowing effect of excessive follicular development, especially in the secondary follicles.
Compared with non-obese women with PCOS, obese women with PCOS have various endocrine changes. High BMI is associated with gonadotropin resistance and poor IVF outcome [19,20]. In addition, high BMI has an inhibitory effect on LH [21]. Therefore, GnRH antagonist protocol in obese PCOS women is likely to work differently than in non-obese PCOS women. For this reason, we divided all subjects into non-obese and obese subgroups, and then evaluated the effectiveness of GnRH antagonist MDP-EL in each subgroups. In non-obese subgroup, IVF results and pregnancy outcome were comparable but total dose and days of rhFSH were significantly fewer in the antagonist group than in the agonist group. Similar results were also observed in obese subgroup. Our results implies that GnRH antagonist MDP-EL is effective not only in non-obese PCOS patients with theoretically higher LH levels but also in obese PCOS patients.
In conclusion, GnRH antagonist MDP-EL can provide similar IVF outcome with fewer dose and days of rhFSH administered in both non-obese and obese PCOS patients, compared with GnRH agonist LP. Moreover, GnRH antagonist MDP-EL can reduce the incidence of severe OHSS. These findings suggest that the GnRH antagonist MDP-EL can be more cost-effective, more patient-friendly and safer than GnRH agonist LP in PCOS patients. Therefore, GnRH antagonist protocol may be the protocol of choice for COS in PCOS patients undergoing IVF-ET, independent of BMI.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Conway GS, Honour JW, Jacobs HS. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol (Oxf) 1989;30:459–470. doi: 10.1111/j.1365-2265.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 3.Homburg R, Berkowitz D, Levy T, Feldberg D, Ashkenazi J, Ben-Rafael Z. In vitro fertilization and embryo transfer for the treatment of infertility associated with polycystic ovary syndrome. Fertil Steril. 1993;60:858–863. doi: 10.1016/s0015-0282(16)56287-6. [DOI] [PubMed] [Google Scholar]
- 4.Homburg R, Levy T, Berkovitz D, Farchi J, Feldberg D, Ashkenazi J, et al. Gonadotropin-releasing hormone agonist reduces the miscarriage rate for pregnancies achieved in women with polycystic ovarian syndrome. Fertil Steril. 1993;59:527–531. doi: 10.1016/s0015-0282(16)55794-x. [DOI] [PubMed] [Google Scholar]
- 5.Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod. 1993;8:959–964. doi: 10.1093/oxfordjournals.humrep.a138174. [DOI] [PubMed] [Google Scholar]
- 6.Borm G, Mannaerts B The European Orgalutran Study Group. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. Hum Reprod. 2000;15:1490–1498. doi: 10.1093/humrep/15.7.1490. [DOI] [PubMed] [Google Scholar]
- 7.Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, Diedrich K, et al. European Cetrorelix Study Group. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. Hum Reprod. 2000;15:526–531. doi: 10.1093/humrep/15.3.526. [DOI] [PubMed] [Google Scholar]
- 8.Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update. 2006;12:651–671. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- 9.Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod Biomed Online. 2007;14:640–649. doi: 10.1016/s1472-6483(10)61059-0. [DOI] [PubMed] [Google Scholar]
- 10.Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–880. doi: 10.1016/s0015-0282(02)04920-8. [DOI] [PubMed] [Google Scholar]
- 11.Elkind-Hirsch KE, Webster BW, Brown CP, Vernon MW. Concurrent ganirelix and follitropin beta therapy is an effective and safe regimen for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2003;79:603–607. doi: 10.1016/s0015-0282(02)04696-4. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum Reprod. 2004;19:1993–2000. doi: 10.1093/humrep/deh375. [DOI] [PubMed] [Google Scholar]
- 13.Kim CH, Lee YJ, Hong SH, Kim SH, Chae HD, Kang BM. Efficacy of a GnRH antagonist during early and late controlled ovarian hyperstimulation period in women with polycystic ovary syndrome undergoing IVF-ET. Hum Reprod. 2004;19:i104–i105. [Google Scholar]
- 14.Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT) Hum Reprod. 2010;25:683–689. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- 15.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44:430–440. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Messinis IE, Milingos SD. Current and future status of ovulation induction in polycystic ovary syndrome. Hum Reprod Update. 1997;3:235–253. doi: 10.1093/humupd/3.3.235. [DOI] [PubMed] [Google Scholar]
- 18.Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: a meta-analysis. Reprod Biomed Online. 2006;13:628–638. doi: 10.1016/s1472-6483(10)60652-9. [DOI] [PubMed] [Google Scholar]
- 19.Fedorcsák P, Dale PO, Storeng R, Tanbo T, Abyholm T. The impact of obesity and insulin resistance on the outcome of IVF or ICSI in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:1086–1091. doi: 10.1093/humrep/16.6.1086. [DOI] [PubMed] [Google Scholar]
- 20.Bellver J, Melo MA, Bosch E, Serra V, Remohí J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88:446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- 21.Pagán YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91:1309–1316. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]