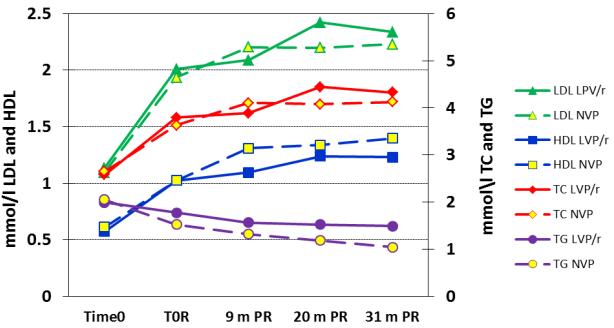
Figure 1.
Mean lipid values among 195 HIV-infected children who achieved viral suppression with ritonavir-boosted lopinavir (LPV/r)-based treatment and then were randomized to either continue on the LPV/r-based regimen (control group) or to switch to a NVP-based regimen (switch group). Mean values are shown prior to starting any treatment (time 0), at the time of randomization when suppressed and at nine (time 0R), 20 and 31 months post-randomization (PR).