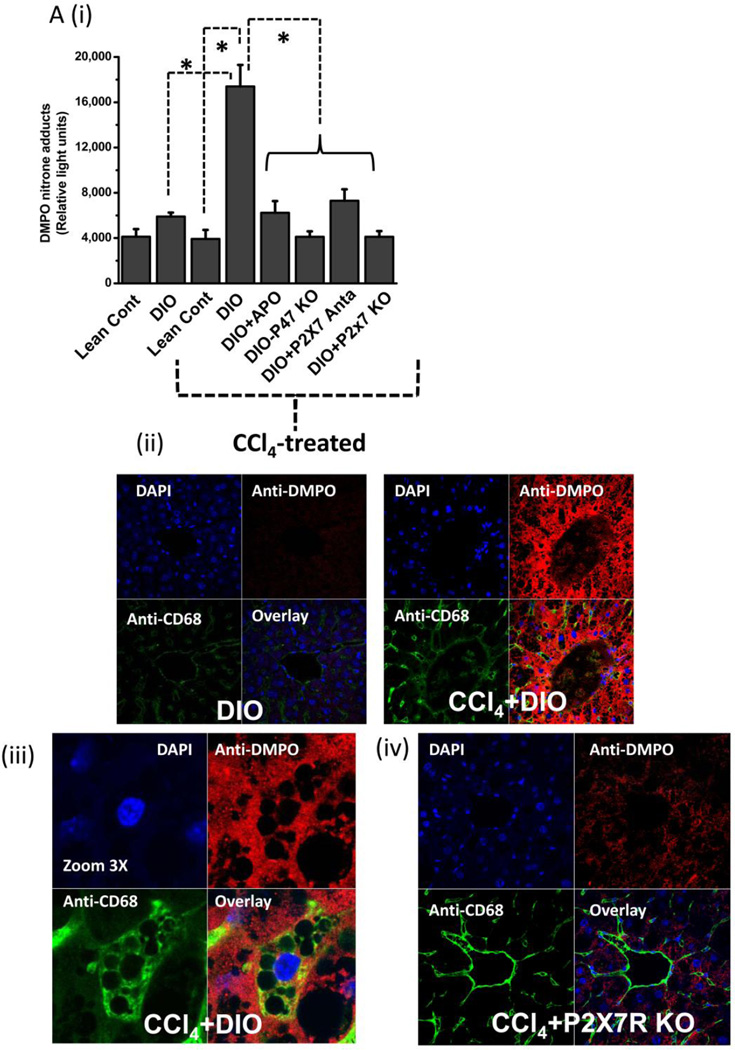
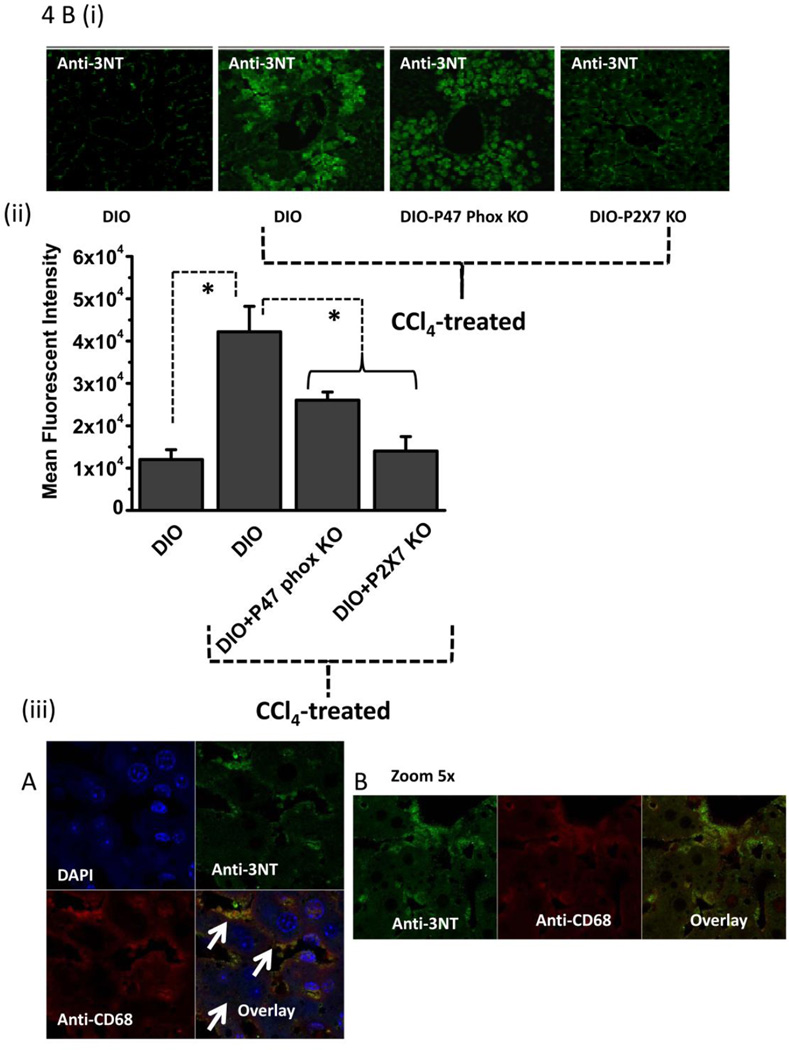
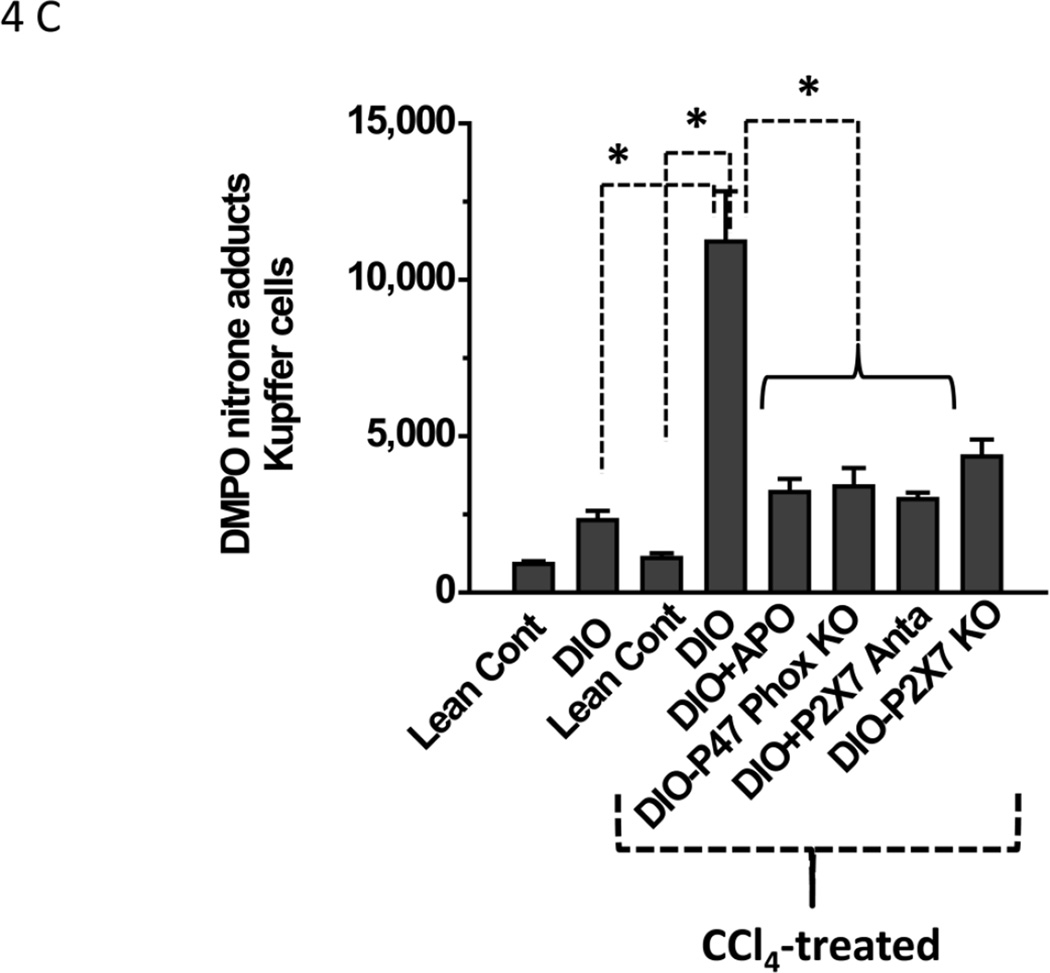
Figure 4.
Metabolic oxidative stress forms protein radicals and post translational tyrosine nitration in Kupffer cells in NADPH oxidase and P2X7 receptor-dependent mechanisms. A. Formation of protein radical adducts in intact liver sections and homogenates using ELISA and confocal microscopy at 24 h post CCl4 administration in DIO mice, high fat-fed P47 phox knockout mice and high fat fed P2X7r knockout mice. (i) DMPO-nitrone adducts in liver homogenates as assayed by ELISA . (ii) Liver sections showing DMPO-nitrone adducts (red) in CD68+ve Kupffer cells (green) in DIO mice ( left panel), and CCl4-treated DIO mice (right panel).(iii) Representative magnified image of Kupffer cell containing DMPO-nitrone adducts in CCl4-treated DIO mice. (iv). Representative image of localization of DMPO-nitrone adducts in P2X7 receptor-deficient mouse liver. B. Formation of nitrotyrosine adducts in Kupffer cells. (i) Confocal image of 3-nitrotyrosine staining in mouse liver sections at 24 h post CCl4 administration. (ii) Mean fluorescent intensities of 3-nitrotyrosine immunoreactivity in liver slices. (iii) Representative magnified images of CCl4-treated DIO mouse liver showing nitrotyrosine adducts (green) in sinusoidal Kupffer cells (red). A and B shows different magnifications. C. DMPO-nitrone adducts in isolated Kupffer cells trans-cultured with hepatocytes. * Significantly different (P<0.05).