Figure 4.
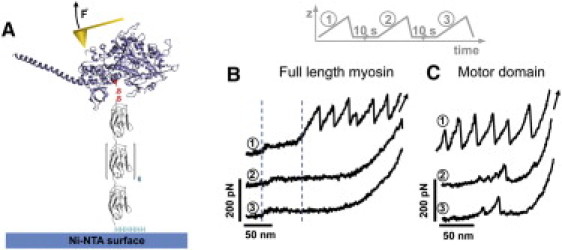
Mechanical fingerprint of derivatized full-length myosin and S1 fragment (motor domain). (A) The motor domain (blue) was derivatized with a mechanical reporter, a tandem repeat I27 polyprotein (gray) carrying an N-terminal cysteine residue and a C-terminal His6 tag. Note that the (I27)8 is both a handle reacting at the reactive cysteines in S1 and a reporter of unfolding and refolding. Coupling was achieved via the reactive cysteines (SH1/SH2) within the motor domain. The handle introduces a means of site-specific attachment via the His6 tag. (B and C) Single molecules of derivatized full-length myosin (B) or S1 (C) were subjected to repeated cycles of mechanical stretching, separated by a waiting time of 10 s at zero force (inset). The I27 sawtooth pattern is apparent in the initial unfolding trace (1), but absent in the second (2) and third (3) traces, indicating that the unfolded motor domain interferes with refolding of the I27 modules. The characteristic low-force plateau stemming from the rod (between dashed lines) is visible in all traces in A, indicating the rod folds as an independent entity and is not affected by the motor domain.
