Figure 5.
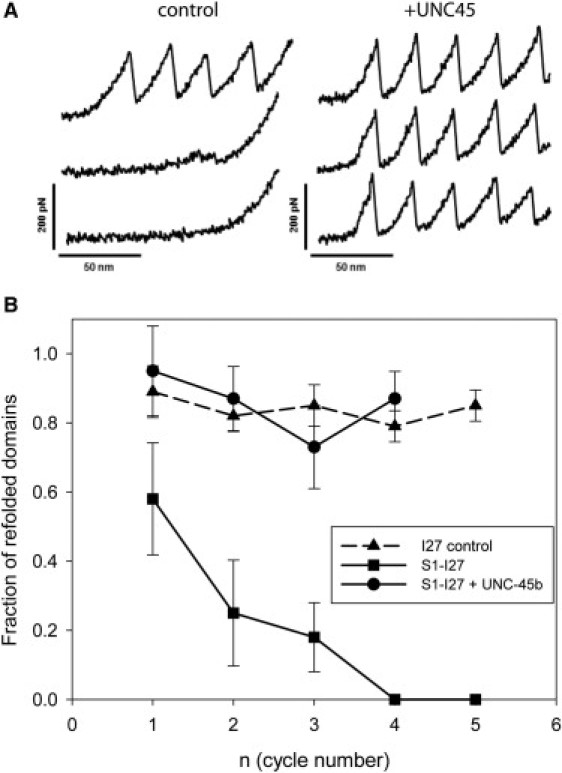
UNC-45b facilitates refolding of the titin mechanical reporter. (A) Single molecules of derivatized S1 were subjected to repeated cycles of unfolding and relaxation to zero force. In the absence of UNC-45b (control), the I27 sawtooth pattern is observed only in the initial unfolding cycle, indicating that the unfolded motor domain interferes with the refolding of the I27 modules. In the presence of 1 μM UNC-45b (right panel), full recovery of folded I27 domains is observed. This result indicates that the I27 domains function as a sensor for motor domain proper folding. UNC-45b prevents the interference between the motor domain and the I27 units, presumably by binding to the myosin motor domain. (B) Plot of the fraction of titin I27 domains as a function of refolding cycles. Single molecules of either the I27 polyprotein (triangles; 18 molecules) or derivatized S1 in the absence of chaperone (squares; 8 molecules) and in the presence of 1 μM UNC-45b (circles; 12 molecules) were subjected to repeat cycles of unfolding. The error bars correspond to the standard deviations.
