Figure 4.
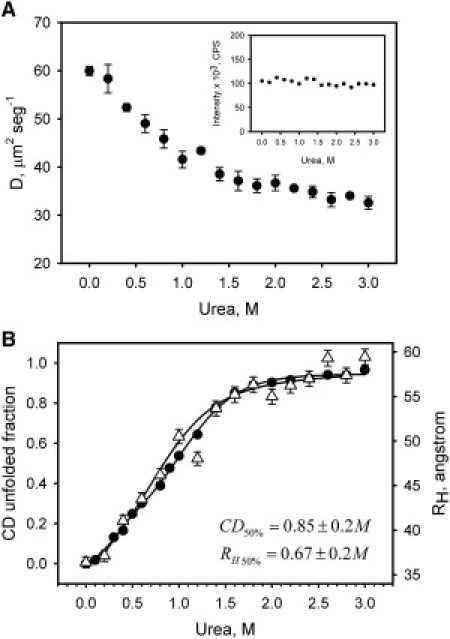
Urea-induced unfolding of FtsZ-Alexa488 conjugates at 100 nM of protein concentration monitored by FCS in 50 mM potassium phosphate buffer, pH 6.5, at room temperature. (A) The diffusion coefficient of FtsZ-Alexa488 conjugates as a function of urea concentration obtained from autocorrelation functions. The inset shows the fluorescence intensity as a function of urea concentration. (B) FtsZ-Alexa488 unfolding data from FCS measurements plotted together with unfolding data from CD measurements of FtsZ at 1 μM. FCS unfolding data are plotted as the hydrodynamic radius (RH) calculated with the Stokes-Einstein equation from diffusion coefficients as described in Materials and Methods. The solid lines represent the two-state fits of the experimental data, and the inset displays the urea concentration required to obtain 50% of denaturation in each case. The error bars represent the SD of three measurements.
