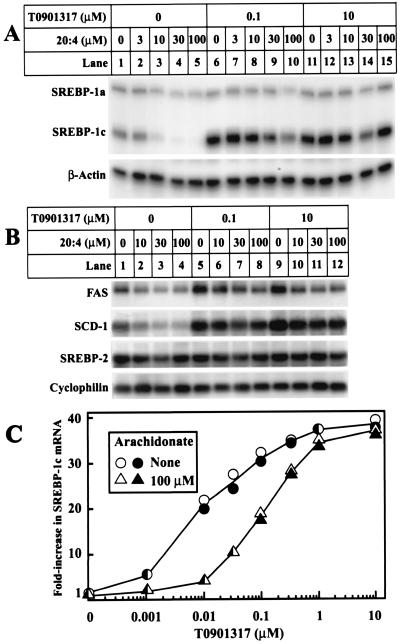
Figure 3.
Suppression of mRNAs encoding SREBP-1c and two target genes by varying concentrations of arachidonate in the absence and presence of T0901317. On day 0, FTO-2B cells were set up in medium A containing 5% FCS. (A and B) On day 3, cells were switched to medium B containing the indicated concentration of sodium arachidonate and T0901317. All dishes of cells received 0.1% BSA and 0.1% DMSO. After incubation for 6 h, the total RNA from two dishes was pooled and isolated. (A) Aliquots of total RNA (20 μg) were hybridized for 10 min at 68°C to the indicated 32P-labeled cRNA probe. RNase-protected fragments were separated by gel electrophoresis and exposed to film at −80°C for 8 h (SREBP-1a and -1c) or 6 h (β-actin). (B) Aliquots of total RNA (20 μg) were subjected to electrophoresis and Northern blot hybridization with the indicated 32P-labeled probe, and filters were exposed to film at −80°C for 3 h (FAS), 4 h (SCD-1), or 8 h (SREBP-2 and cyclophilin). (C) On day 2, cells were switched to medium B containing 50 μM compactin and 50 μM sodium mevalonate. On day 3, cells were refed with the same medium containing the indicated concentrations of T0901317 and arachidonate. All dishes of cells received 0.1% BSA and 0.1% DMSO. After incubation for 6 h, total RNA from duplicate dishes was separately isolated, aliquots (20 μg) were analyzed as described in A, and the bands for SREBP-1c were quantified with a Fuji Bio-Imaging analyzer and normalized to the β-actin control. Values for each of the duplicate incubations are shown.