Abstract
Context:
Traditional medicine, which is more available and affordable for the poor uses medicinal plants for the treatment and management of various ailments, including the sickle cell disease (SCD). About 24 million Nigerians are carriers of this sickled cell gene, while approximately 2.4 million are SCD patients. Moringa oleifera Lam. (Moringaceae) possesses high nutritional value and has been used in folklore medicine to treat various ailments related to pain and inflammation. Chemical, pharmacological and pharmacognostical applications of Moringa oleifera have been reported.
Objective:
This study investigated the antisickling potential of polar and non-polar extracts of the seed, flower and leaf of Moringa oleifera for the first time.
Materials and Methods:
Using crude methanol extract, aqueous extract, ethyl acetate and butanol, the in vitro antisickling activities of Moringa oleifera fractions, were evaluated using erythrocyte cells deoxygenated with 2% sodium metabisulphite. p-Hydroxybenzoic acid and normal saline were employed as positive and negative controls.
Results:
Phytochemical screening revealed the presence of saponins, free anthraquinones, and alkaloids. Extracts of the seed and flower demonstrated a higher (P<0.05) antisickling activity in comparison to the leaf extract. The leaf extract, as well as those of the seed and flower, equally demonstrated a (P<0.05) reversal of sickled erythrocytes.
Discussions and Conclusions:
These findings suggest that Moringa oleifera may play a role in the management of SCD, by incorporation of its fractions into recipes. More extensive biological evaluations and further studies will be necessary for the chemical characterization of the antisickling principles.
Keywords: Moringa oleifera, non-polar fractions, phytochemicals, polar extracts, sickle cell disease
Moringa oleifera Lam. (Moringaceae) (MO), commonly known as drumstick or horseradish, is native to the Sub-Himalaya tracts of India, Pakistan, Bangladesh, Central America, Afghanistan, and Africa.[1,2] MO, which is rich in vegetable oil and high in nutritional values, is used in Asia as a vegetable and medicinal plant. This is attributed to the presence of proteins, vitamins, and various phenolic compounds in the oil.[2] Structures of some of the previously isolated phytoconstituents from MO plant[1] are shown in Figure 1. Nevertheless, all parts of the Moringa tree are edible and have been consumed for many years by humans.
Figure 1.
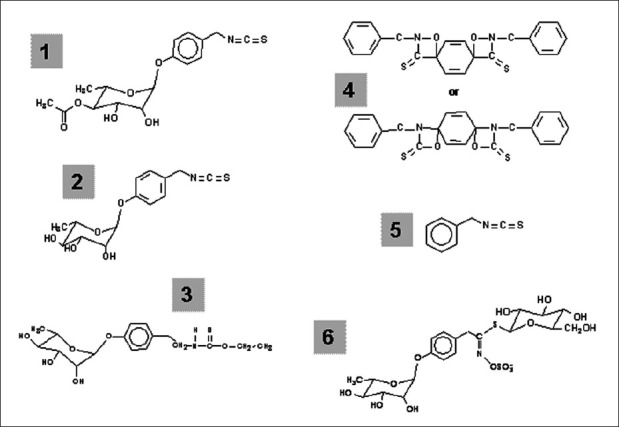
Structures of selected phytochemicals from Moringa spp.: [1] 4-(4’-O-acetyl-α-L-rhamnopyranosyloxy)benzyl isothiocyanate, [2] 4-(-L-rhamnopyranosyloxy)benzyl isothiocyanate, [3] niazimicin, [4] pterygospermin, [5] benzyl isothiocyanate, and [6] 4-(α-L-rhamnopyranosyloxy)benzyl glucosinolate Fahey[1] (used with permission)
The diverse range of medicinal uses for Moringa oleifera, include its use as an antioxidant,[3] anticarcinogenic,[4] anti-inflammatory, antispasmodic, diuretic,[5] antiulcer, antibacterial, antifungal[6] and its antinociceptive[7] properties, as well as its wound healing ability has been demonstrated.[8] Additionally, the root bark has been used as an analgesic, alexeteric, antihelminthic, and treatment for heart complaints, as well as for eye diseases, inflammation and dyspepsia.[9,10]
Elujoba et al.[11] reported that the use of traditional medicine in the treatment and management of an array of diseases in the African continent is likely to continue due to Africa's socio-cultural, socio-economic heritage, lack of basic healthcare, and support for the rural population. Sickle cell disease (SCD) has become a challenge in the African continent with about 89% of global sufferers of SCD. About twenty-five (25%) of the SCD patients in the world are in Nigeria.[12] Hence, this confers on Nigeria the highest population of sickle cell disease patients in the world. Clinical manifestations of SCD are diverse and fall into three major categories, namely, anemia, pain related and organ failure.[13]
A report by Ndiaye et al.[14] stated that Moringa oleifera is used in African folk medicine to treat rheumatic and articulary pain, but to the best of our knowledge, the use of Moringa oleifera in the treatment and management of SCD has not been reported. This study is the first of its kind and the findings of this investigation are reported herein. However, considering its use in folk medicine as an analgesic and for the treatment of pain and inflammation, investigating its antisickling potential would have a well-founded justification. Therefore, this work focused on assessing the phytochemical and antisickling potential of methanol extract (ME), aqueous extract (AqE), ethyl acetate (EA) and butanol (BU) of the seeds, flowers and leaves of Moringa oleifera.
Materials and Methods
Plant collection and preparation
The leaves, seed and flowers of Moringa oleifera were collected between January and March, 2009 from Ibadan, Oyo state and Sagamu, Ogun State, Nigeria. The plant was identified by a plant taxonomist, Mr. Felix, at the Forestry Research Institute of Nigeria in Ibadan, where a voucher specimen was deposited. The studied plant parts were air dried at room temperature (28±2°C) and was powdered using mortar and pestle. The powdered samples were then stored in airtight containers and properly labeled for further analyses.
Phytochemical tests
Powdered samples for each of the plant parts were used to test for alkaloids, saponins, and tannins. Phytochemical analyses were carried out using standard procedures.[15,16]
Extraction
Each of the dried powdered material (500 g) was macerated with methanol (2 l) for 7 days in large amber bottles and filtered. The filtrate was concentrated using a rotatory evaporator, under reduced pressure. For the aqueous extract, dried powdered materials were macerated in distilled water for 3 days, using the same proportion (500 g) used for the methanol extraction. The filtrates obtained were then partitioned successively with ethylacetate and butanol. The aqueous, ethylacetate, and butanol fractions were concentrated using the rotatory evaporator. Thereafter, each of the different fractions was serially diluted with normal saline (0.9% NaCl), to give 10 and 20 mg/ ml which were used for the antisickling assay.
Solvents and chemicals
All the solvents (namely methanol, ethanol, butanol, chloroform, and ethyl acetate) and chemical reagents used in this study were of analytical grade and were procured from SIGMA Chemicals Co. Dorset, UK; and BDH Chemicals Limited Poole, England, respectively.
Blood collection and preparation
Blood (5 ml) was obtained in duplicate from a SCD volunteer by venipuncture after informed consent was given in accordance with approved University protocols. The volunteer, who was in steady state, was a confirmed sickle cell disease patient (HbSS) attending the Haematology Day Care Unit of the Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria. Blood was collected in sodium EDTA bottles and the content thoroughly mixed by gentle rolling of the bottle. The blood sample was centrifuged to remove serum, leaving the packed erythrocytes, which had been washed with normal saline, as described by Egunyomi et al.[17]
Bioassay of plant extracts for antisickling activity
Bioassay of both crude methanol extract and the aqueous fraction of the three plant materials for antisickling activity were carried out using two approaches. These were the inhibition of sickling (antisickling) approach and the reversal of sickled erythrocytes. Antisickling activity of the extracts/fractions was evaluated using a modified method.[18] The washed erythrocytes (0.5 ml) were mixed with 0.5 ml of each concentration of the test extracts/fractions, in uncovered test tubes.
Samples were then taken from the different mixtures and the remainder, incubated at 37°C, for 3 h while shaking occasionally. Five drops of sodium metabisulphite (2%) were added to the mixture and this was mixed thoroughly and sealed with liquid paraffin. Duplicate samples were taken from the different mixtures at 0 min, followed by the incubation of the systems at 37°C. Additional samples were taken at 30 min intervals, until four further readings were recorded. Smear preparations and counting of sickled and unsickled cells were achieved by following the method, described by Egunyomi et al.[17] Two types of controls were employed in this bioassay. p-Hydroxybenzoic acid (5 mg/ml) was used as a positive control, while normal saline was used as a negative control. The percentage inhibition of sickling was calculated using the formula given in Moody et al.,[19] while the sickling reversal activity of the different plant extracts/fractions was evaluated by using the procedure described by Oduola et al.[20]
The washed erythrocytes (0.5 ml) were again mixed with 0.5 ml of freshly prepared sodium metabisulphite (2%) in a clean test tube and incubated at 37°C, for 30 min. A drop of this mixture was viewed under the microscope. Equal volumes of normal saline/extract/fraction were added to the blood-metabisulphite mixture in a different test tube and incubated at 37°C, for another 30 min. Samples were taken at 0 min and at 30 min intervals for up to 2 h. The earlier procedure described was again used for smear preparations and the counting of sickled and unsickled cells.[17]
Statistical analysis
Data obtained were expressed as means. The statistical significance of differences was assessed using analysis of variance (ANOVA). A two-tailed P value of less than 0.05 was considered to be statistically significant.
Results
The yields from the aqueous fraction are seed (6.3%), leaf (17.9%), and flower (42.4%). Extractive yield in methanol, ethyl acetate and butanol for seed are 9.7%, 2.2%, and 3.2% while for leaf are 20.1%, 1.3%, and 0.6%, respectively. It can be inferred that higher yield was observed for the aqueous and methanol fractions. Table 1 shows the results for screened phytochemicals of the investigated plant materials. Saponins were detected in all the three plant organs studied. Free anthraquinone was found only in the flower and alkaloids only, in the seed. The results of the antisickling assay are presented in Tables 2–4. p-Hydroxybenzoic acid being the positive control and as expected exhibited antisickling activity. Other tested extracts/fractions also exhibited antisickling activity. Although the antisickling activities of all the tested extracts/fractions compared favorably with that exhibited by PHBA, the exhibited antisickling activities were found to be concentration dependent.
Table 1.
Screened phytochemicals of leaves, flowers and seeds of Moringa oleifera

Table 2.
Antisickling effect (% inhibition of sickling) of methanol, aqueous, butanol, and ethylacetate fractions of Moringa oleifera seed
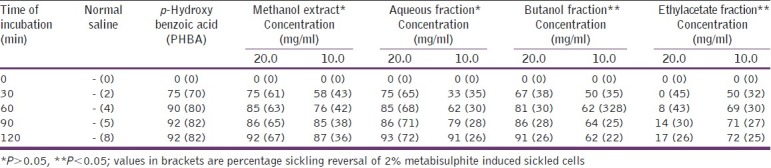
Table 4.
Antisickling effect (% inhibition of sickling) of methanol and aqueous fraction of Moringa oleifera flower
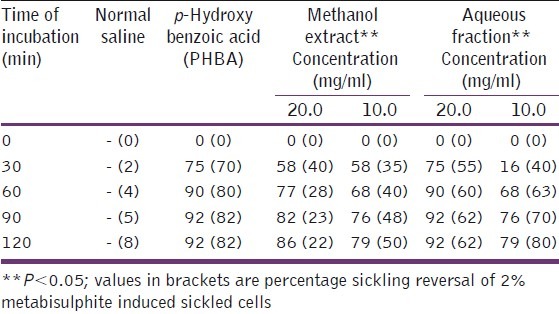
Table 3.
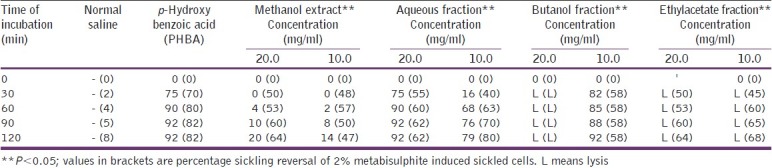
Antisickling effect (% inhibition of sickling) of methanol, aqueous, butanol, and ethylacetate fractions of Moringa oleifera leaf
Results of antisickling bioassay for both AqE and ME of MO seed showed that there was no significant difference (P>0.05) between the antisickling activity exhibited at 10 and 20 mg/ ml concentrations, at the end of the 2 h incubation period. However, it is noteworthy that at the lower concentration, the antisickling activity gradually increased before it peaked towards the end of incubation.
Mpiana et al. worked at a concentration range of between 0 and 10 mg/ml for the antisickling activity of anthocyanins from Ocimum basilicum.[21] Antisickling activity of the seeds’ butanol fraction was significantly (P<0.05) higher at 20 mg/ ml concentration and that of ethylacetate fraction, at 10 mg/ml concentration. The EA fraction of MO leaf (at both tested concentrations), as well as the BU fraction (at 20 mg/ml), caused lysis of the blood.
Extracts of the seed and flower fractions, demonstrated a significantly higher (P<0.05) antisickling activity than the leaf extract. The aqueous fraction equally exhibited a significantly higher (P<0.05) antisickling activity as the crude methanol extract, also as shown. The seeds’ aqueous extracts exhibited a higher percentage reversal of sickling of all the tested parts. However, sickling reversal was more pronounced at the highest tested concentration (20 mg/ml). Nonetheless, this is in contrast with the pattern of sickling reversal exhibited by the flowers’ aqueous extract, which at a lower concentration of 10 mg/ml, gradually increased until it peaked after an incubation period of 2 h. Contrarily, at 20 mg/ ml after an initial increase within 30 min incubation, the percentage sickling reversal decreased until the end of 120 min of incubation. Both butanol and ethylacetate fractions of MO seed and leaf had insignificant (P>0.05) abilities to reverse sickled erythrocytes.
Discussion
The contribution of phytochemicals to the antisickling activity of any medicinal plant used in the management of SCD is not in doubt, as many reports have attributed the antisickling properties of such plants to their innate phytochemicals. For instance, zanthoxylol, a butyric acid derivative and 1-hydroxylbenzoic acid, which were isolated from Fagara zanthoxyloides Lam. (Rutaceae) have been suggested to be responsible for the antisickling activity of this plant.[18] Moringa oleifera has been reported to contain a rich store of elements like zinc, which possesses antisickling activity, as well as organic acids. Drumstick leaves are also rich sources of flavonols, such as, kaempferol and 3’-OMe quercetin. A flavone, acacetin, and a glycoflavone 4-OMe Vitexin were also detected. Phenolic acids that have been identified, include melilotic acid, p-coumaric acid, and vanillic acid,[22] which could be responsible for the exhibited antisickling activities.
Phytochemical examination of the herbal formula extract, Ajawaron (used for SCD management in Nigeria) whose main constituent is the root of Cissus populnea Guill and Perr. (Vitaceae) was found to contain anthraquinones, steroidal and cardiac glycosides, while alkaloids and tannins were absent.[19] This study consequently lends credence to the earlier affirmed position considering the phytochemical compositions of the investigated plant parts vis a viz their corresponding exhibited antisickling activity.
Both the seed and flower fractions of Moringa oleifera exhibited a significantly higher antisickling activity, when compared with the leaf extract. This could be attributed to the fact that the latter had only saponins, while the former duo had anthraquinones and alkaloids in addition to saponins. The fact that both butanol and ethylacetate fractions of MO leaf could cause lysis of erythrocytes depending on the concentration used, is an important factor that will caution against the inclusion of MO leaf in the recipes for SCD treatment. It has been reported that the mode of preparation of traditional recipes, as stipulated by the herb seller, was by decoction with clean water.[17] The observed significantly higher (P<0.05) antisickling activity of aqueous extract in this study, supports this, and it is believed that oxidative damage to cells is responsible for the activation of KCl-co-transport in sickled erythrocytes.[23] The sickled cell erythrocytes being fragile and dehydrated require that minerals and antioxidants be constantly supplied to maintain hydration of the cells and membrane integrity.
Consequently, the contribution of micronutrients and the antioxidative properties of some plants, to their antisickling properties have been investigated. Such plants include, aged garlic,[24] Mormodica charantia L. (Curcubitaceae)[25] and Cymbrogon citratus Stapf. (Gramineae).[26] Incidentally, the antioxidative properties of Moringa oleifera had been reported in literature.[27,28] It then becomes probable that the observed antisickling properties of Moringa oleifera seed and flower fractions in this study could possibly be due to its innate antioxidants and phytochemicals.
In this study, the crude methanol extract of Moringa leaf had an insignificant (P>0.05) antisickling activity. Paradoxically, its activity in reversing the sickled cell was highly significant (P<0.05). Four cations (K+, Na+, Ca2+, and Mg2+) have reportedly come into prominence in modulating the ionic pathways involved in the dehydration process.[29] Additionally, it was reported that the ensuing electrolyte imbalances are triggered by diffusional and osmolytical activities. Previous reports have evaluated the mineral contents of Moringa oleifera leaves.[30,31] On understanding that cations such as K+, Na+, Ca2+, and Mg2+ (which are implicated in the process of sickling) may be important parameters in sickle cell management;[32] the involvement of some of these cationic contents of Moringa leaf in the modulation of the ionic pathways of the sickled SS erythrocytes, being responsible for the reversal of sickling observed in the present study is plausible. However, this should be further investigated.
Fahey[1] in his study asserted that Moringa oleifera is native to the Sub-Himalaya tracts of India, Pakistan, Bangladesh and Afghanistan but the fact that Nigeria carries 25% of the 89% SCD sufferers in Africa,[12] could be the reason that the plant is being lately propagated in Nigeria. None of the traditional recipes that are used in SCD management in Nigeria contained Moringa oleifera as a constituent to the best of our knowledge. Therefore, this study which is the first to test the antisickling effects of a common edible plant in Sub-Saharan Africa where sickle cell disease is most prevalent presents a platform to explore the use of Moringa oleifera for the management of sickle cell disease patients.
Conclusions
Findings from the present study have indicated for the first time the antisickling potentials of the seed and flower of Moringa oleifera. This suggests the plant could be a valuable source of antisickling agents. The fact that Moringa oleifera exhibits antiurolithiatic properties[33] may also advance its use in SCD patients, particularly to enhance renal function. Further studies will be necessary for the chemical characterization of the active antisickling principles; as well as extensive biological evaluations to generate in vivo availability data, and to elucidate the necessary mechanisms involved in order to improve our understanding of such plants for the management of sickle cell disease.
Acknowledgments
The authors acknowledge the contributions of Professor P.O. Olatunji (Head, Department of Haematology), Dr. Wura Fred-Jaiyesimi (Head, Department of Pharmacognosy), Olabisi Onabanjo University, Sagamu, Nigeria and personal assistance of Mr. Bayo Adeyemi of the Haematology Laboratory, Olabisi Onabanjo University Teaching Hospital, Sagamu, with special thanks. Also, we are most grateful to Dr. Ranti Fayokun for her painstaking assistance in correcting the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fahey JW. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties Part 1: Trees for Life Journal. 2005. [Last accessed on 2011 Aug 07]. Available from: http://www.tfljournal.org/article.php/20051201124931586 .
- 2.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 3.Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Bharali R, Tabassum J, Azad MR. Chemomodulatory effect of Moringa oleifera, Lam on hepatic carcinogen metabolizing enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pacific J Cancer Prev. 2003;4:131–9. [PubMed] [Google Scholar]
- 5.Cáceres A, Saravia A, Rizzo S, Zabala L, De Leon E, Nave F. Pharmacologic properties of Moringa oleifera.2: Screening for antispasmodic, anti-inflammatory and diuretic activity. J Ethnopharmacol. 1992;36:233–7. doi: 10.1016/0378-8741(92)90049-w. [DOI] [PubMed] [Google Scholar]
- 6.Caceres A, Cabrere O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera.1: Preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–6. doi: 10.1016/0378-8741(91)90078-r. [DOI] [PubMed] [Google Scholar]
- 7.Sulaiman MR, Zakaria ZA, Bujarimin AS, Somcent MN, Israf DA, Moin S. Evaluation of Moringa oleifera aqueous extract for antinociceptive and anti-inflammatory activities in animal models. Pharm Biol. 2008;46:838–45. [Google Scholar]
- 8.Rathi BS, Bodhankar SL, Baheti AM. Evaluation of aqueous leaves extract of Moringa oleifera Linn.for wound healing in albino rats. Indian J Exp Biol. 2006;44:898–01. [PubMed] [Google Scholar]
- 9.Nadkarni KM. 1-II. Bombay: Popular Prakashan Private Limited (Popular Press); 1976. India Material Medica; pp. 1–968. [Google Scholar]
- 10.Chopra R, Chopra IC, Handa KL, Kapur LD. 2nd ed. 1982. Drugs of India; pp. 569–608. (610, 680). [Google Scholar]
- 11.Elujoba AA, Odeleye OM, Ogunyemi CM. Traditional medicine development for medical and dental primary health care delivery system in Africa. J Trad Compl Alt Med. 2005;2:46–61. [Google Scholar]
- 12.Ameh SJ, Obodozie OO, Afolabi EK, Oyedele EO, Ache TA, Onanuga CE, et al. Some basic requirements for preparing an antisickling herbal medicine-NIPRISAN. Afr J Pharm Pharmacol. 2009;3:259–64. [Google Scholar]
- 13.Ohnishi ST, Ohnishi T, Ogunmola GB. Sickle cell anaemia: A potential nutritional approach for a molecular disease. Nutrition. 2000;16:330–8. doi: 10.1016/s0899-9007(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 14.Ndiaye M, Dieye AM, Mariko F, Tall A, Sall-Diallo A, Faye B. Contribution to the study of the anti-inflammatory activity of Moringa oleifera (Moringaceae) Dakar Med. 2002;47:210–2. [PubMed] [Google Scholar]
- 15.Adesanya SA, Sofowora EA. Biological standardization of Zanthoxylum roots for antisickling activity. Planta Med. 1983;48:27–33. doi: 10.1055/s-2007-969873. [DOI] [PubMed] [Google Scholar]
- 16.Harborne JB. 3rd ed. London: Chapman and Hill; 1998. Phytochemical methods: A guide to modern techniques of plant analysis; p. 279. [Google Scholar]
- 17.Egunyomi A, Moody JO, Eletu OM. Antisickling activities of two ethnomedicinal plant recipes used for the management of sickle cell anaemia in Ibadan, Nigeria. Afr J Biotechnol. 2009;8:20–5. [Google Scholar]
- 18.Sofowora EA, Isaac-Sodeye WA, Ogunkoya LO. Isolation and characterization of an antisickling agent from the root of Fagara zanthoxyloides. In: Sofowora EA, editor. African Medicinal Plants. Proceedings of a conference; 1979. pp. 89–97. [Google Scholar]
- 19.Moody JO, Ojo OO, Omotade OO, Adeyemo AA, Olumese PE, Ogundipe OO. Antisickling potential of a Nigerian herbal formula (Ajawaron HF) and the major plant component (Cissus populnea L. CPK) Phytother Res. 2003;17:1173–6. doi: 10.1002/ptr.1323. [DOI] [PubMed] [Google Scholar]
- 20.Oduola T, Adeniyi FA, Ogunyemi EO, Bello IS, Idowu TO. Antisickling agent in an extract of unripe pawpaw (Carica papaya): Is it real? Afr J Biotechnol. 2006;5:1947–9. [Google Scholar]
- 21.Mpiana PT, Mudogo V, Ngbolua KN, Tshibangu DS, Shetonde OM, Mbala MB. In vitro antisickling activity of anthocyanins from Ocimum basilicum L. (Lamiaceae) Int J Pharmacol. 2007;3:371–4. [Google Scholar]
- 22.Nambiar VS, Mehta R, Daniel M. Polyphenol content of three Indian green leafy vegetables. J Food Sci Technol. 2005;42:312–5. [Google Scholar]
- 23.Brugnara C. Reticulocyte cellular indices: A new approach in the diagnosis of anaemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci. 2000;37:93–130. doi: 10.1080/10408360091174196. [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi ST, Ohnishi T. In vitro effects of aged garlic extract and other nutritional supplements on sickle cell erythrocytes. J Nutr. 2001;131:1085S–92S. doi: 10.1093/jn/131.3.1085s. [DOI] [PubMed] [Google Scholar]
- 25.Semiz A, Sen A. Antioxidant and chemoprotective properties of Momordica charantia L. (bitter lemon) fruit extract. Afr J Biotechnol. 2007;6:273–7. [Google Scholar]
- 26.Ojo OO, Kabutu FR, Bello M, Babayo U. Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citratus) and green tea (Camella sinensis) in rats. Afr J Biotechnol. 2006;5:1227–32. [Google Scholar]
- 27.Njoku OU, Adikwu MU. Investigation on some physico-chemical, antioxidant and toxicological properties of Moringa oleifera seed oil. Acta Pharmaceutica Zagreb. 1997;47:287–90. [Google Scholar]
- 28.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves. J Agric Food Chem. 2003;51:2144–55. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 29.Brugnara C, De Franceshi L, Alper SL. Inhibition of Ca2+ dependent K+ transport and cell dehydration in Sickle erythrocytes by CLT and other imidazole derivatives. J Clin Invest. 1993;92:520–6. doi: 10.1172/JCI116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pankaja N, Prakash J. Availability of calcium from kilkeerai (Amaranthus tricolor) and drumstick (Moringa oleifera) greens in weaning rats. Nahrung. 1994;38:199–203. doi: 10.1002/food.19940380212. [DOI] [PubMed] [Google Scholar]
- 31.Barminas JT, Charles M, Emmanuel D. Mineral composition of non-conventional leafy vegetables. Plant Foods Hum Nutr. 1998;53:29–36. doi: 10.1023/a:1008084007189. [DOI] [PubMed] [Google Scholar]
- 32.Segun IF, Odukoya AO, Moody JO. Management of sickle cell anaemia in Nigeria with medicinal plants: Cationic evaluation of extracts and possible effects on the efficacy. J Biol Sci. 2006;6:100–102. [Google Scholar]
- 33.Karadi RV, Palkar MB, Gaviraj EN, Gadge NB, Mannur VS, Alagawadi KR. Antiurolithiatic property of Moringa oleifera root bark. Pharm Biol. 2008;46:861–5. [Google Scholar]


