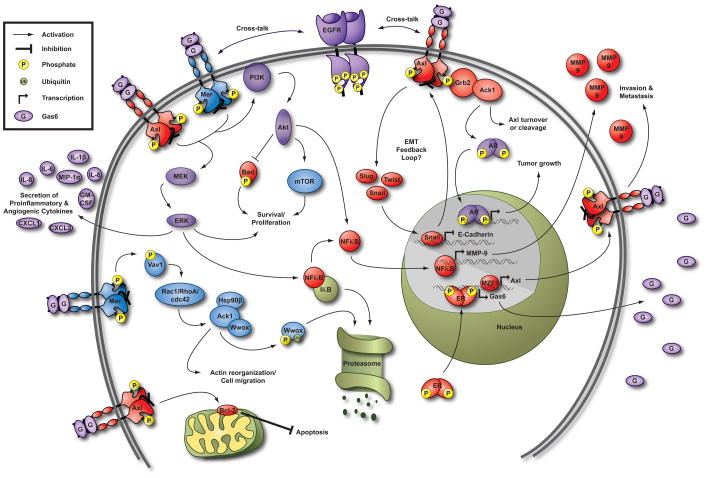
Figure 2. Axl and Mer signaling pathways in cancer cells.
Pathways activated downstream of both Mer and Axl, such as the Phosphatidylinositol 3-kinase (PI3K)/Protein kinase B (Akt) and Mitogen-activated protein kinase (MEK)/Extracellular-signal-regulated kinase (ERK) survival and proliferation pathways, are shown in purple. Blue pathways, including regulation of mammalian target of rapamycin (mTOR) [10], have been described downstream of Mer. Pathways downstream of Axl such as inactivation of Bcl-2-associated death promoter (BAD) via phosphorylation by Akt are shown in red. Mer interacts with, but does not directly phosphorylate, activated cdc42-associated kinase 1 (Ack1). Activation of Ack1 may occur indirectly through the guanine nucleotide-exchange factor Vav1 and cdc42, resulting in degradation of the tumor suppressor protein WW domain containing oxidoreductase (Wwox) and regulation of cell migration [123]. Interestingly, Ack1 also interacts with Axl via Grb2 and may regulate Axl turnover or cleavage [122]. Gas6 stimulates Ack1-dependent phosphorylation of androgen receptor (AR) resulting in proliferation of prostate cancer cells in vitro, presumably via transcriptional regulation of androgen responsive genes [124]. This effect could be mediated by Mer and/or Axl since the cell types evaluated express both receptors. Axl expression is only found in estrogen receptor (ER)-positive patient samples, and ER antagonists reduce Axl expression in breast cancer cells [33, 72]. It has not been determined whether ER binds to the Axl promoter, but Gas6 expression is transcriptionally regulated by ER [75]. See text for explanation of other depicted pathways. Additional signaling pathways have been delineated in non-cancerous cells (reviewed in [17]). Hsp90β, heat shock protein 90β; IκB, inhibitor of kappa-B; Rac1, Ras-related C3 botulinum toxin substrate 1; RhoA, Ras homolog gene family member A.