Abstract
B1 B cells defend against infectious microorganisms by spontaneous secretion of broadly reactive “natural” immunoglobulin that appears in the absence of immunization. Among many distinguishing characteristics, B1 B cells display evidence of activation that includes phosphorylated STAT3. In order to identify the origin of pSTAT3 we examined interleukin-2 receptor (IL-2R) expression on B1 cells. We found that some (about 1/5) B1a cells express the IL-2R α chain, CD25. Although lacking CD122 and unresponsive to IL-2, B1a cells marked by CD25 express increased levels of activated signaling intermediates, interruption of which results in diminished CD25. Further, CD25+ B1a cells contain most of the pSTAT3 found in the B1a population as a whole. Moreover, CD25+ B1a cells express leukemia inhibitory factor receptor (LIFR), and respond to LIF by upregulating pSTAT3. Together, these results define a new subset of B1a cells that is marked by activation-dependent CD25 expression, expresses substantial amounts of activated STAT3, and contains a functional LIFR.
Keywords: B1 cells, CD25, LIF receptor, signaling
Introduction
In mice, B1 cells represent a unique subset of B lymphocytes originally distinguished from the more abundant conventional splenic B cells by expression of the pan-T cell marker, CD5. In addition to CD5, other phenotypic characteristics that identify B1 cells include sIgMhi, sIgDlo, CD23−, and CD43+ (reviewed in Hardy and Hayakawa, 2001; Wortis and Berland, 2001; Berland and Wortis, 2002; Rothstein, 2002). During development, B1 cells appear first after which B2 cell production proceeds while relative B1 cell numbers decline (Hayakawa et al., 1983; Lalor et al., 1989; Hamilton et al., 1994). In adult mice, B1 cells are the predominant lymphocyte population in the peritoneal and pleural cavities, are present in small numbers in the spleen, and are absent in the peripheral blood and lymph nodes (Hayakawa et al., 1983). B1 B cells are capable of self-renewal, giving rise to their own progeny, in contrast to B2 cells which are continually generated in the bone marrow from stem cell precursors (Hayakawa et al., 1986; Kantor et al., 1995), although recently it has been suggested that the B1 cell pool in adult animals admits bone marrow-derived emigrants over time (Duber et al., 2009; Holodick et al., 2009a). Two B1 cell populations exist, B1a cells that express CD5, and B1b cells that lack CD5 but are otherwise phenotypically similar to B1a cells although in some ways functionally distinct (Kantor et al., 1992; Alugupalli et al., 2004). Much of what is known about B1 cells concerns CD5+ B1a cells.
B1 cells contribute to immune protection through spontaneous production of “natural” immunoglobulin that is generated in the absence of specific immunization and accounts for most of the “resting” IgM and a substantial portion of the “resting” IgA found in the serum. B1 cell-derived natural immunoglobulin is critically important in the early defense against, and clearance of, bacterial and viral infections (Briles et al., 1981; Su et al., 1991; Boes et al., 1998; Benedict and Kearney, 1999; Ochsenbein et al., 1999; Baumgarth et al., 2000; Alugupalli et al., 2004). Natural immunoglobulin differs from B2 cell-derived immunoglobulin in being more germline like – as a result of minimal N-region addition and somatic hypermutation – and is repertoire-skewed reflecting antigen-driven selection (Hardy et al., 1989; Pennell et al., 1989; Gu et al., 1990). The rules governing immunoglobulin production by B1 cells appear to differ from those that regulate immunoglobulin production by B2 cells, in that B1 cell immunoglobulin secretion is much less dependent on Blimp-1 and IRF4 than is that of B2 cells, although a role for Blimp-1 in B1 cell immunoglobulin secretion has been suggested (references Lin et al., 2003; Tumang et al., 2005; Klein et al., 2006; Savitsky and Calame, 2006; Holodick et al., 2010).
Aside from constitutive immunoglobulin secretion, B1 cells manifest a number of distinctive features. In comparison to B2 cells, they present antigen more efficiently and unlike B2 cells, they induce naïve CD4 T cells to become Th17 cells (Zhong et al., 2007a,b). Moreover, B1 cells respond mitogenically to phorbol ester in the absence of a calcium ionophore whereas B2 cells do not, and in contrast to B2 cells, B1 cells fail to proliferate, nor to activate NF-κB, in response to BCR engagement (Rothstein and Kolber, 1988a, 1988b; Morris and Rothstein, 1993). The unique mitogen responses of B1 cells are reflected in distinct alterations of cyclins D2 and D3 (Tanguay et al., 1999, 2001).
Beyond these functional characteristics, the nature of B1 cells remains uncertain. B1 cells express a number of genes, proteins, and transcription factors differently than B2 cells (Fischer et al., 2001; Wong et al., 2002; Frances et al., 2006, 2007). This fits with the idea that B1 cells represent a separate B cell lineage, a notion supported by the recent identification of a distinct B220lo/−CD19+ B1 cell progenitor (Herzenberg, 2000; Montecino-Rodriguez et al., 2006). On the other hand, B1 cells appear to develop in relation to the strength and nature of BCR signaling (Arnold et al., 1994; Casola et al., 2004; Hardy, 2006). This fits with the idea that B1 cells represent a particular differentiation state of a single B cell lineage (Cong et al., 1991; Wang and Clarke, 2004). The finding that B1 cells show evidence of prior or ongoing activation, such as CD44 expression and elevated baseline pERK (Murphy et al., 1990; Wong et al., 2002), would seem to support the latter notion, and we have concluded in other work that B1 cells experience continual signaling (Holodick et al., 2009b). On the other hand, B1 cells lack some facets of activation, such as CD69 expression or elevated baseline nuclear NF-κB or c-myc expression (Morris and Rothstein, 1993; Wang et al., 1995; Tumang et al., 2004). Particularly perplexing has been the constitutive expression by B1 cells of activated, tyrosine phosphorylated STAT3 (Karras et al., 1997).
CD25 [interleukin-2 receptor (IL-2R) α chain] is a 55-kDa glycoprotein, which along with CD122 (β chain) and CD132 (common γ-chain) forms the high affinity receptor for IL-2 (Minami et al., 1993). In B cells, CD25 expression is first detected during the pre-B cell stage of development after which expression declines as newly formed B cells migrate to the spleen to form the mature B2 cell compartment (Rolink et al., 1994). In mature B2 cells, CD25 is re-expressed as an activation marker in response to antigenic encounter in an NF-κB/c-Rel dependent manner (Muraguchi et al., 1985; Tumang et al., 1998).
Interleukin-2 receptor signaling has been shown to induce phosphorylation and activation of STAT3 (Nielsen et al., 1994; Brunn et al., 1995; Frank et al., 1995). In order to elucidate the origin of pSTAT3 in B1a cells, and in view of our finding that B1 cells show evidence of continual signaling, we considered the possibility that B1 cells might express CD25/IL-2R and that IL-2R signaling might account for B1 cell activated STAT3. In fact, we did find that some, but not all, B1 cells express CD25, although not as a complete, IL-2-responsive receptor. We further found that CD25 expression divides B1 cells into two populations, one of which (CD25+) contains pSTAT3 and activated signaling intermediates, expresses leukemia inhibitory factor receptor (LIFR), and responds to LIF, whereas the other (CD25−) for the most part does not.
Materials and Methods
Animals
Male BALB/cByJ, C57BL/6, and C.B17-Prkdcscid mice at 8–14 weeks of age were obtained from The Jackson Laboratory. All experiments were approved by the Institutional Animal Care and Use Committee, and mice were cared for and handled in accordance with National Institutes of Health and institutional guidelines.
B cell purification and culture
Sort-purified peritoneal B1 were obtained on the basis of CD5 and B220 staining (CD5+B220lo). Splenic follicular (FO) B2 and marginal zone (MZ) B2 cells were obtained on the basis of CD23 and CD21 expression. Splenic T cells were obtained on the basis of CD5 and B220 staining (CD5+B220−). Sort-purified B1 cells were further subdivided on the basis of CD25 expression. Populations were reanalyzed for purity by flow cytometry and subsets determined to be >98% pure. Sort-purified B cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Gene expression
RNA was prepared from B cells using Ultraspec reagent (BiotecX), was DNase treated, and was reverse transcribed using iScript (BioRad). Gene expression was then assessed by real-time PCR (Stratagene) using the following primers (forward/reverse): β2-microglobulin (CCCGCCTCACA TTGAAATCC/GCGTATGTATCAGTCTCAGTGG); LIFR; ATGGC ACATTGACTCGCCTC/GCACGAAGGGTATTGCCGAT), SOCS3 (CCCGCTTCGACTGTGTACTCA / GAGGTCGGCTCAGTACCA GC), and CD122 (CACAGGCCAGCTGCTTCAC/AGGCATTGGG CAGATGGAA).
Protein expression
Sort-purified cells were extracted and extracted proteins were immunoblotted as previously described (Tumang et al., 2005). Membranes were developed using the ECL Western Blotting Analysis System from Amersham Biosciences. As a protein loading control, blots were stripped and reprobed with anti-actin Ab.
Phosphoflow analysis
Intracellular phosphospecific flow cytometry and fluorescent cell barcoding were carried out as previously described (Holodick et al., 2009b). Flow cytometric analysis was performed using a BD Biosciences LSR II.
Reagents
Fluorescently labeled anti-B220, anti-CD5, anti-CD23, anti-CD21, anti-CD69, and anti-CD25 (clone PC61) antibodies for flow cytometry and cell sorting were obtained from BD Biosciences. F(ab′)2 fragments of goat anti-mouse IgM for B cell stimulation in vitro were obtained from Jackson Immunoresearch. Recombinant LIF and IL-6 for B cell stimulation in vitro were obtained from R&D Systems. LY294002 and Syk inhibitor [(3-(1-Methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide] were obtained from Calbiochem. Antibodies directed against tyrosine phosphorylated (705) STAT3 and STAT3 for immunoblotting were obtained from Cell Signaling Technology. Fluorescent antibodies directed against tyrosine phosphorylated Syk, PLCγ2, and STAT3 for phosphoflow analysis were obtained from BD Biosciences. Anti-LIFR antibody for immunoblotting was obtained from Santa Cruz Biotechnology. Anti-actin antibody was obtained from Sigma-Aldrich.
Results
CD25 is expressed on a subset of naïve peritoneal B1 cells
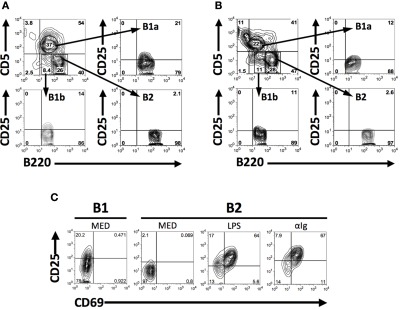
CD25 is expressed on B2 cells as an activation marker (Tumang et al., 1998), and naïve B1 cells show evidence of continual activation (Holodick et al., 2009b), which together raise the possibility that B1 cells might constitutively express CD25. To determine whether B1 cells express CD25, we analyzed B cell populations by flow cytometry following immunofluorescent staining. Results are shown in Figure 1. We found that some (about 1/5), but not all, naïve BALB/c peritoneal B1 (B1a) cells expressed CD25, as did a smaller number of B1b cells (about 1/10). In contrast, few if any splenic B2 cells (data not shown) or peritoneal B2 cells (Figure 1A) expressed CD25. B1a cell expression of CD25 was not limited to BALB/c mice, as some, albeit fewer, C57BL/6 B1a cells also expressed CD25 in contrast to C57BL/6 B2 cells (Figure 1B). Thus, CD25 expression divides B1 cells into two separate populations. We compared the level of CD25 expression on BALB/c peritoneal B1a cells with the level of expression on splenic B2 cells after stimulation by LPS or anti-Ig for 2 days. Results are shown in Figure 1C. We found that CD25 expression by CD25+ (CD69−) peritoneal B1a cells encompassed a range similar to that of CD25+CD69+ splenic B2 cells, although the mean fluorescence intensity of the latter outweighed the former.
Figure 1.
CD25 is expressed on a subset of naïve peritoneal B1 cells. (A,B) Freshly isolated BALB/c (A) and C57BL/6 (B) peritoneal washout cells were immunofluorescently stained for surface expression of B220, CD5, and CD25. Gates were set to identify B1a (B220lo, CD5+), B1b (B220lo, CD5−), and B2 (B220+, CD5−) cells among B220+ B cells and expression of CD25 was assessed for each population. Representative results from one of seven (A) and three (B) comparable experiments are shown. (C) Freshly isolated BALB/c B220loCD5+ B1a cells, and BALB/c splenic B220+CD5− B2 cells cultured in medium (MED) or stimulated with LPS (25 μg/ml) or with F(ab′)2 fragments of goat anti-mouse IgM (15 μg/ml) for 2 days, were immunofluorescently stained for CD25 and CD69. CD25 mean fluorescence intensity values (above background isotype staining) for CD25+ B1a cells and for CD25+CD69+B2 cells after stimulation with LPS and anti-Ig were, respectively, 53, 123, and 189. One of three comparable experiments is shown.
CD25 expression by B1a cells is associated with, and depends on, activated signaling intermediates
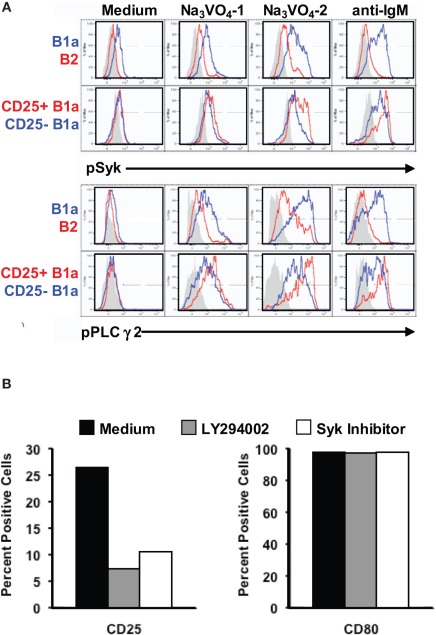
The finding that some B1a cells do, and other B1a cells do not, express the activation-related marker, CD25, raises the possibility that CD25+ B1a cells are signaling more intensely than CD25− B1a cells. To address this issue, we examined the phosphorylation status of BCR-triggered signaling intermediates in sort-purified B cell populations by phosphoflow analysis, utilizing barcoding after fixation and permeabilization to facilitate experimentation (Krutzik and Nolan, 2006). To enhance detection of phosphorylated intermediates, we added sodium orthovanadate to unstimulated B cells for 1 or 2 min, to block dephosphorylation (Holodick et al., 2009b). Results are shown in Figure 2A. We found that B1a cells accumulated substantial amounts of intracellular pSyk and pPLCγ2 after exposure to sodium orthovanadate for 2 min, whereas B2 cells showed a much smaller increase, as previously reported (Holodick et al., 2009b). Importantly, within the B1a population, CD25+ B1a cells showed evidence of much more pSyk and pPLCγ2 than did CD25− B1a cells. The same patterns held true when B cells were stimulated by anti-IgM (in the absence of Na3VO4). Here again B1a cells increased pSyk and pPLCγ2 to higher levels than did B2 cells, and CD25+ B1a cells were more responsive than CD25− B1a cells. Thus, CD25 expression marks a B1a subset that is more active in terms of constitutive, ongoing phosphorylation of signaling intermediates, and is more responsive in terms of BCR-triggered signaling.
Figure 2.
CD25 expression by B1a cells is associated with, and depends on, activated signaling intermediates. (A) Four populations of B cells were sort-purified: peritoneal B1a cells (“B1a” in blue in upper panels), splenic follicular B2 cells (“B2” in red in upper panels), CD25+ peritoneal B1a cells (“CD25+ B1a” in red in lower panels), and CD25− peritoneal B1a cells (“CD25− B1a” in blue in lower panels). B cells were cultured in medium alone (“Medium”), and with Na3VO4 at 10 mM for 1 or 2 min, as indicated, or with F(ab′)2 fragments of goat anti-mouse IgM at 15 μg/ml for 4 min (“anti-IgM”). Cells were then harvested, fixed, permeabilized, and examined for intracellular tyrosine phosphorylated Syk (“pSyk”) and tyrosine phosphorylated PLCγ2 (“pPLCγ2”) by phosphoflow analysis. Isotype control antibody staining is displayed in gray. Representative results from one of three comparable experiments are shown. (B) Sort-purified B1a cells were cultured for 18 h with either LY294002 or with Syk inhibitor after which expression of CD25 (left panel) and of CD80 (right panel) was assessed by flow cytometry. Mean results from three independent experiments are shown along with lines indicating SE of the means.
The elevated level of phosphorylated signaling intermediates in B1a cells that express the activation-related marker, CD25, suggests the possibility that B1a CD25 expression depends on enhanced signaling. To address this possibility, we examined sort-purified B1a cells for CD25 expression before and after inhibition of PI-3K by treatment with LY294002 and inhibition of Syk by treatment with a peptide Syk inhibitor, for 18 h. Over this period of time these inhibitors do not affect B cell viability (Holodick et al., 2009b). Results are shown in Figure 2B. We found that interference with PI-3K or with Syk substantially abrogated CD25 expression by B1a cells. In contrast, and as a control, inhibition of neither PI-3K nor Syk produced a reduction in expression of the B7 molecule CD80. Thus, B1a expression of CD25 is specifically dependent on enhanced activation of signaling intermediates.
CD25+ B1a cells display elevated levels of phosphorylated tyrosine705 STAT3
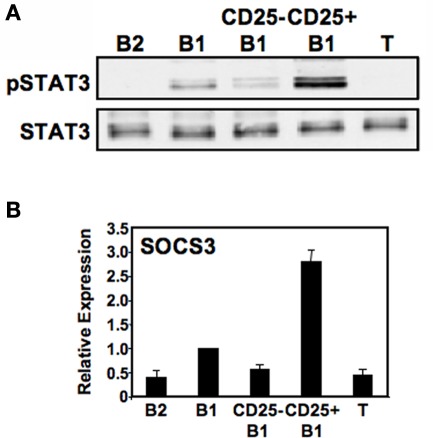
Because IL-2 is capable of activating STAT3, CD25 expression by some B1a cells raises the possibility that the CD25+ B1a subset harbors all of the constitutively phosphorylated STAT3 attributed to B1 cells as a whole (Karras et al., 1997). To address this possibility, we examined sort-purified lymphocyte populations by Western blotting for the activated form of STAT3 (pTyr705STAT3). Results are shown in Figure 3A. We found that, as previously described, peritoneal B1 cell lysates contained pTyr705STAT3 whereas splenic B2 and T cell lysates did not. Importantly, we found that among B1a cells, the large majority of pTyr705STAT3 was located specifically within the CD25+ B1a fraction. To verify the relative enrichment of CD25+ B1a cells for pTyr705STAT3 we examined expression of the STAT3-regulated gene, SOCS3, by real-time PCR amplification of reverse-transcribed RNA obtained from sort-purified B cell populations. Results are shown in Figure 3B. We found that, as expected, B1a cells expressed levels of SOCS3 much higher than the levels expressed by B2 cells. Importantly, we found that among B1a cells, the large majority of SOCS3 transcripts were located specifically within the CD25+ B1a fraction. Thus, a select subset of B1a cells that expresses the IL-2Rα chain (CD25) and contains elevated levels of activated signaling intermediates also expresses the bulk of phosphorylated, functionally active STAT3 that was previously known to be upregulated in B1a cells as a whole.
Figure 3.
CD25+ B1a cells display elevated levels of phosphorylated tyrosine705 STAT3. Five lymphocyte populations were sort-purified: splenic follicular B2 cells (“B2”), peritoneal B1a cells (“B1”), CD25− peritoneal B1a cells (“CD25− B1”), CD25+ peritoneal B1a cells (“CD25+ B1”), and splenic T cells (“T”). (A) Protein extracts were prepared from each of these populations and immunoblotted for expression of pTyr705STAT3 (“pSTAT3”). Blots were stripped and reprobed for expression of total STAT3 as a loading control. Representative results from one of two comparable experiments are shown. (B) RNA was prepared from each of these populations, reverse transcribed, and evaluated for expression of SOCS3 by real-time PCR, normalized to β2-microglobulin. Expression by each population relative to peritoneal B1a cells is shown as the mean of six experiments with lines indicating the SE of the means.
However, it is unlikely that CD25 and pSTAT3 are directly connected, inasmuch as expression of CD25 was not accompanied by expression of CD122 (IL-2Rβ chain) transcript (Figure 4) or surface protein (data not shown), which were essentially absent in B1 and B2 cell populations, as contrasted with activated T cells. Further, there was no response of CD25+ B1a cells to IL-2 as shown by the failure of IL-2 to produce tyrosine phosphorylation of STAT5 (data not shown). These results indicate that CD25 marks a pTyr705STAT3-containing subset of peritoneal B1 cells but does not function as a complete, cytokine-responsive receptor.
Figure 4.
CD25 expression on a subset of B1a cells is not accompanied by expression of CD122. Five lymphocyte populations were sort-purified: splenic follicular B2 cells (“B2”), peritoneal B1a cells (“B1”), CD25− peritoneal B1a cells (“CD25− B1”), CD25+ peritoneal B1a cells (“CD25+ B1”), and splenic T cells (“T”). B cell populations were unstimulated whereas T cells were stimulated by ConA at 5 μg/ml for 2 days. RNA was prepared, reverse transcribed, and evaluated for expression of CD122 by real-time PCR, normalized to β2-microglobulin. Expression by each population relative to peritoneal B1a cells is shown as the mean of six experiments with lines indicating the SE of the means.
CD25+ B1a cells express LIF receptor
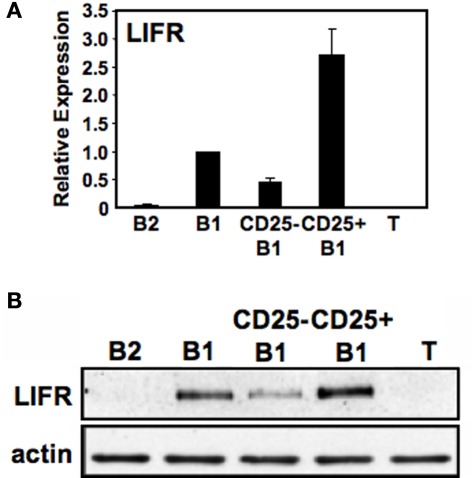
The association between CD25 expression and phosphorylated STAT3, combined with the lack of a direct mechanism connecting the two, raises the possibility that another STAT3-active cytokine receptor segregates with CD25+ B1a cells. In previous work with embryonic stem cells, LIF-induced self-renewal was shown to depend on STAT3 (Niwa et al., 1998); inasmuch as B1 cells display the property of self-renewal along with activated STAT3, we considered the possibility that LIF and LIFR might play a role in B1a pSTAT3 expression. Inasmuch as immunofluorescent reagents are not available to detect murine LIFR, we first examined sort-purified B cell populations for LIFR gene expression by real-time PCR. Results are shown in Figure 5A We found that B1a cells expressed LIFR transcripts whereas B2 cells did not. Importantly, we found that the level of LIFR gene expression was much greater in CD25+ B1a cells than in CD25− B1a cells. To verify the relative enrichment of CD25+ B1a cells for LIFR expression, we evaluated LIFR protein expression by Western blotting. Results are shown in Figure 5B. Much like LIFR gene expression, we found that B1a cells expressed LIFR protein whereas B2 cells (and T cells) did not; importantly, we found that the level of LIFR was much greater in CD25+ B1a cells than in CD25− B1a cells. Thus, LIFR segregates with CD25 expression among peritoneal B1a cells, much like phosphorylated STAT3 and activated signaling intermediates.
Figure 5.
CD25+ B1a cells express LIF receptor (LIFR). Five lymphocyte populations were sort-purified: splenic follicular B2 cells (“B2”), peritoneal B1a cells (“B1”), CD25− peritoneal B1a cells (“CD25− B1”), CD25+ peritoneal B1a cells (“CD25+ B1”), and splenic T cells (“T”). (A) RNA was prepared from each of these populations, reverse transcribed, and evaluated for expression of LIFR by real-time PCR, normalized to β2-microglobulin. Expression by each population relative to peritoneal B1a cells is shown as the mean of six experiments with lines indicating the SE of the means, with the exception of splenic T cells for which n = 3. (B) Protein extracts were prepared from each of these populations and immunoblotted for expression of “LIFR”. Blots were stripped and reprobed for expression of total beta actin (actin) as a loading control. Representative results from one of three comparable experiments are shown.
CD25+ B1a cells respond to LIF
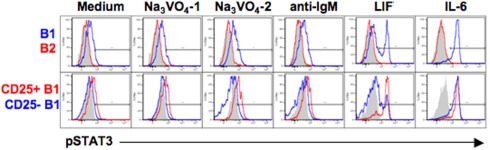
The CD25+ B1a cell LIFR might mediate STAT3 phosphorylation; on the other hand, the failure of CD25 to mediate IL-2 signaling in these cells raises the possibility that LIFR might be similarly indolent. To address this issue, we examined the phosphorylation status of STAT3 after LIF treatment in sort-purified B cell populations by phosphoflow analysis. Results are shown in Figure 6. We found that, at baseline and as expected, B1a cells contained more pTyr705STAT3 than B2 cells, and CD25+ B1a cells contained more pSTAT3 than CD25− B1a cells; these relationships were both accentuated by treatment with Na3VO4. Importantly, we found that addition of LIF (in the absence of Na3VO4) produced a marked increase in pSTAT3 in B1a cells (but not in B2 cells), resulting in a bimodal distribution, suggesting the presence of responsive and non-responsive populations. Further examination of separated CD25+ and CD25− B1a cells revealed that the increase in pSTAT3 induced by LIF occurred predominantly within the CD25+ B1a population. As a control, IL-6 treatment of these B cell populations produced an increase in pSTAT3 that was equally shared by the CD25+ and CD25− B1a populations. Thus, LIF specifically affects LIFR-expressing CD25+ B1a cells indicating that the LIFR is functional in this B1a subset.
Figure 6.
CD25+ B1a cells respond to LIF. Four populations of B cells were sort-purified: peritoneal B1a cells (“B1” in blue in upper panel), splenic follicular B2 cells (“B2” in red in upper panels), CD25+ peritoneal B1a cells (“CD25+ B1” in red in lower panels), and CD25− peritoneal B1a cells (“CD25− B1” in blue in lower panels). B cells were cultured in medium alone (“Medium”), with Na3VO4 at 10 mM for 1 or 2 min, as indicated, with F(ab′)2 fragments of goat anti-mouse IgM at 15 μg/ml for 4 min (“anti-IgM”), with recombinant LIF at 10 ng/ml for 5 min, or with recombinant IL-6 at 10 ng/ml for 5 min. Cells were then harvested, fixed, permeabilized, and examined for intracellular tyrosine phosphorylated STAT3 (“pSTAT3”) by phosphoflow analysis. Isotype control antibody staining is displayed in gray. Representative results from one of three comparable experiments are shown. The displayed results were obtained at the same time as the results shown in Figure 2A.
Discussion
The work described herein identifies a novel subset of B1 cells that expresses the high affinity IL-2 receptor α chain, CD25. Despite comprising only one-fifth of peritoneal B1a cells, the CD25+ population accounts for two key characteristics previously attributed to the whole population of peritoneal B1a cells: constitutive expression of pSTAT3 (along with attendant expression of SOCS3); and, continual activation of signaling intermediates. It is unclear why CD25− B1a cells are deficient in these features, but this finding raises the possibility that CD25+ and CD25− B1a cells differ in origin, development, BCR specificity, and/or function.
One function in particular that differs between CD25+ and CD25− B1a cells relates to LIF signaling, the receptor for which is here shown to be expressed preferentially by CD25+ B1a cells. LIFR expression to our knowledge has not been previously reported on any B cell subset. Further, LIFR on CD25+ B1a cells constitutes a functioning receptor that responds to LIF with an increase in STAT3 phosphorylation. This suggests a role for LIF in the baseline level of pSTAT3 previously shown to be elevated in B1a cells and here shown to segregate with CD25+ B1a cells. Although the net result of LIFR and pSTAT3 expression by CD25+ B1a cells remains uncertain, these molecules have been reported to contribute powerfully to processes of activation, expansion, differentiation, and immunoregulation (Taupin et al., 1998; Bowman et al., 2000; Bromberg and Darnell, 2000; Calo et al., 2003; Dimitriou et al., 2008), implying a distinct physiology for CD25+ as opposed to CD25− B1a cells. In particular, LIF and STAT3 promote self-renewal and pluripotency of embryonic stem cells (Niwa et al., 1998; Cartwright et al., 2005), suggesting that B1a cell self-renewal may be focused on the CD25+ population that is activated, presumably, as a result of self-antigen recognition. It may be speculated that this would promote the maintenance of a population of B1a cells specialized for production of immunoglobulin that serves to homeostatically bind and dispose of cellular debris (Binder and Silverman, 2005). Of note, SOCS3 inhibits pSTAT3 and LIFR signaling (Naka et al., 1997; Starr et al., 1997; Auernhammer and Melmed, 2001; Yoshimura et al., 2007), suggesting a complex interplay among these components that in the context of B cells takes place solely within a minor subset of B1a cells that expresses CD25.
Although LIFR expression correlates with signaling in response to LIF, the index marker for the CD25+ B1a subset, CD25 (IL-2Ra), does not constitute a complete receptor and does not mediate signaling in response to IL-2 as it does in activated B2 cells, due to the lack of CD122 (IL-2Rβ; Muraguchi et al., 1985; Minami et al., 1993). On the other hand, CD25 expression appears to reflect activation in B1a cells as it does in B2 cells, because CD25+ B1a cells preferentially manifest continual activation of signaling mediators, as previously described for B1a cells in general, and because interference with signalosome mediators produces a rapid decline in CD25 expression. Of note, however, the stability of CD25 expression over long periods of time in vivo after adoptive transfer (unpublished observations) suggests that CD25 does not reflect a temporary stage of, or transient event in, B1a cells, but rather corresponds to a chronic condition of activation. Our previous work suggests that continual activation of signaling mediators in B1a cells is BCR-driven, presumably on the basis of antigen, or self-antigen, recognition. In this scenario a consequence of continual signaling, upregulation of CD25, would also be determined by BCR antigen specificity, which as an unchanging characteristic is consistent with CD25 persistence. Analysis of CD25+ and CD25− B1a immunoglobulins showed a trend toward more N-less (and thus more germline like) sequences in the former (unpublished observations); however, this did not reach the level of significance and it will be necessary to examine antigen recognition rather than antibody structure to elucidate the origin of B1a continual signaling and CD25 expression. Of note, no difference in spontaneous antibody secretion has been noted between CD25+ and CD25− B1 cells (unpublished observations).
As a positive control for activation of signaling intermediates B cell antigen receptors were polyclonally crosslinked with anti-IgM. In B1a cells, this led to an increase in pSyk and pPLCγ2, that was more marked in CD25+ as compared to CD25− B1a cells. These results recapitulate our earlier finding (Morris and Rothstein, 1994) that BCR crosslinking in B1 cells yields normal induced phosphorylation of PLCγ2 that, however, fails to produce full enzymatic activation. In light of the failure of BCR crosslinking in B1 cells to produce NF-κB activation or mitogenic stimulation, phosphorylation of signaling intermediates as shown here and elsewhere (Wong et al., 2002) emphasizes that BCR signaling in B1 cells is not indolent, just different. The recent report that SOCS3 can interfere with NF-κB activation (Bruun et al., 2009) suggests another explanation for the early termination of BCR signaling in B1 cells (Rothstein and Kolber, 1988a,b; Morris and Rothstein, 1993).
In sum, CD25+ B1a cells represent a minor B1 cell population that preferentially embodies the known B1 cell characteristics of continual signaling and activated STAT3, and is here shown to be the sole B cell population that expresses LIFR and responds to LIF. The latter may contribute to constitutive expression of pSTAT3 in B1 cells.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by United States Public Health Service grants AI029690 and AI060896 awarded to Thomas L. Rothstein by the National Institutes of Health.
Abbreviations
IMGT, ImMunoGeneTics; LIF, leukemia inhibitory factor; LIFR, LIF receptor; PLC, phospholipase C; Syk, spleen tyrosine kinase.
References
- Alugupalli K. R., Leong J. M., Woodland R. T., Muramatsu M., Honjo T., Gerstein R. M. (2004). B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Arnold L. W., Pennell C. A., McCray S. K., Clarke S. H. (1994). Development of B-1 cells: segregation of phosphatidylcholine-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 179, 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auernhammer C. J., Melmed S. (2001). The central role of SOCS-3 in integrating the neuro-immunoendocrine interface. J. Clin. Invest. 108, 1735–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Herman O. C., Jager G. C., Brown L. E., Herzenberg L. A., Chen J. (2000). B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192, 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C. L., Kearney J. F. (1999). Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 10, 607–617 10.1016/S1074-7613(00)80060-6 [DOI] [PubMed] [Google Scholar]
- Berland R., Wortis H. H. (2002). Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253–300 10.1146/annurev.immunol.20.100301.064833 [DOI] [PubMed] [Google Scholar]
- Binder C. J., Silverman G. J. (2005). Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin. Immunopathol. 26, 385–404 [DOI] [PubMed] [Google Scholar]
- Boes M., Prodeus A. P., Schmidt T., Carroll M. C., Chen J. (1998). A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188, 2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T., Garcia R., Turkson J., Jove R. (2000). STATs in oncogenesis. Oncogene 19, 2474–2488 10.1038/sj.onc.1203527 [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. (1981). Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153, 694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J., Darnell J. E., Jr. (2000). The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 10.1038/sj.onc.1203476 [DOI] [PubMed] [Google Scholar]
- Brunn G. J., Falls E. L., Nilson A. E., Abraham R. T. (1995). Protein-tyrosine kinase-dependent activation of STAT transcription factors in interleukin-2- or interleukin-4-stimulated T lymphocytes. J. Biol. Chem. 270, 11628–11635 [DOI] [PubMed] [Google Scholar]
- Bruun C., Heding P. Eo., Ronn S. G., Frobose H., Rhodes C. J., Mandrup-Poulsen T., Billestrup N. (2009). Suppressor of cytokine signalling-3 inhibits Tumor necrosis factor-alpha induced apoptosis and signalling in beta cells. Mol. Cell. Endocrinol. 311, 32–38 [DOI] [PubMed] [Google Scholar]
- Calo V., Migliavacca M., Bazan V., Macaluso M., Buscemi M., Gebbia N., Russo A. (2003). STAT proteins: from normal control of cellular events to tumorigenesis. J. Cell. Physiol. 197, 157–168 [DOI] [PubMed] [Google Scholar]
- Cartwright P., McLean C., Sheppard A., Rivett D., Jones K., Dalton S. (2005). LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132, 885–896 10.1242/dev.01670 [DOI] [PubMed] [Google Scholar]
- Casola S., Otipoby K. L., Alimzhanov M., Humme S., Uyttersprot N., Kutok J. L., Carroll M. C., Rajewsky K. (2004). B cell receptor signal strength determines B cell fate. Nat. Immunol. 5, 317–327 [DOI] [PubMed] [Google Scholar]
- Cong Y. Z., Rabin E., Wortis H. H. (1991). Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int. Immunol. 3, 467–476 [DOI] [PubMed] [Google Scholar]
- Dimitriou I. D., Clemenza L., Scotter A. J., Chen G., Guerra F. M., Rottapel R. (2008). Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol. Rev. 224, 265–283 [DOI] [PubMed] [Google Scholar]
- Duber S., Hafner M., Krey M., Lienenklaus S., Roy B., Hobeika E., Reth M., Buch T., Waisman A., Kretschmer K., Weiss S. (2009). Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood 114, 4960–4967 10.1182/blood-2009-04-218156 [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Solt L. A., Hastings W. D., Yang K., Gerstein R. M., Nikolajczyk B. S., Clarke S. H., Rothstein T. L. (2001). Splenic and peritoneal B-1 cells differ in terms of transcriptional and proliferative features that separate peritoneal B-1 from splenic B-2 cells. Cell. Immunol. 213, 62–71 [DOI] [PubMed] [Google Scholar]
- Frances R., Tumang J. R., Kaku H., Gurdak S. M., Rothstein T. L. (2006). B-1 cells express transgelin 2: unexpected lymphocyte expression of a smooth muscle protein identified by proteomic analysis of peritoneal B-1 cells. Mol. Immunol. 43, 2124–2129 [DOI] [PubMed] [Google Scholar]
- Frances R., Tumang J. R., Rothstein T. L. (2007). Extreme skewing of annexin II and S100A6 expression identified by proteomic analysis of peritoneal B-1 cells. Int. Immunol. 19, 59–65 [DOI] [PubMed] [Google Scholar]
- Frank D. A., Robertson M. J., Bonni A., Ritz J., Greenberg M. E. (1995). Interleukin 2 signaling involves the phosphorylation of Stat proteins. Proc. Natl. Acad. Sci. U.S.A. 92, 7779–7783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Forster I., Rajewsky K. (1990). Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 9, 2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. M., Lehuen A., Kearney J. F. (1994). Immunofluorescence analysis of B-1 cell ontogeny in the mouse. Int. Immunol. 6, 355–361 [DOI] [PubMed] [Google Scholar]
- Hardy R. R. (2006). B-1 B cell development. J. Immunol. 177, 2749–2754 [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Riblet R. J., Hayakawa K. (1989). A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 142, 3643–3651 [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. (2001). B cell development pathways. Annu. Rev. Immunol. 19, 595–621 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. (1986). Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur. J. Immunol. 16, 450–456 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. (1983). The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 157, 202–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L.A. (2000). B-1 cells: the lineage question revisited. Immunol. Rev. 175, 9–22 [PubMed] [Google Scholar]
- Holodick N. E., Repetny K., Zhong X., Rothstein T. L. (2009a). Adult BM generates CD5( B1 cells containing abundant N-region additions. Eur. J. Immunol. 39, 2383–2394 10.1002/eji.200838920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick N. E., Tumang J. R., Rothstein T. L. (2009b). Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Mol. Immunol. 46, 3029–3036 10.1016/j.molimm.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick N. E., Tumang J. R., Rothstein T. L. (2010). Immunoglobulin secretion by B1 cells: differential intensity and interferon response factor 4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur. J. Immunol. 40, 3007–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A. B., Stall A. M., Adams S., Herzenberg L. A. (1992). Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. U.S.A. 89, 3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A. B., Stall A. M., Adams S., Watanabe K., Herzenberg L. A. (1995). De novo development and self-replenishment of B cells. Int. Immunol. 7, 55–68 [DOI] [PubMed] [Google Scholar]
- Karras J. G., Wang Z., Huo L., Howard R. G., Frank D. A., Rothstein T. L. (1997). Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocytes. J. Exp. Med. 185, 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Casola S., Cattoretti G., Shen Q., Lia M., Mo T., Ludwig T., Rajewsky K., Dalla-Favera R. (2006). Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 7, 773–782 [DOI] [PubMed] [Google Scholar]
- Krutzik P. O., Nolan G. P. (2006). Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat. Methods 3, 361–368 [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Herzenberg L. A., Adams S., Stall A. M. (1989). Feedback regulation of murine Ly-1 B cell development. Eur. J. Immunol. 19, 507–513 [DOI] [PubMed] [Google Scholar]
- Lin K. I., Tunyaplin C., Calame K. (2003). Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol. Rev. 194, 19–28 [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. (1993). The IL-2 receptor complex: its structure, function, and target genes. Annu. Rev. Immunol. 11, 245–268 10.1146/annurev.iy.11.040193.001333 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Leathers H., Dorshkind K. (2006). Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 [DOI] [PubMed] [Google Scholar]
- Morris D. L., Rothstein T. L. (1993). Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J. Exp. Med. 177, 857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L., Rothstein T. L. (1994). Decreased surface IgM receptor-mediated activation of phospholipase C gamma 2 in B-1 lymphocytes. Int. Immunol. 6, 1011–1016 [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. (1985). Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J. Exp. Med. 161, 181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. P., Kolber D. L., Rothstein T. L. (1990). Elevated expression of Pgp-1 (Ly-24) by murine peritoneal B lymphocytes. Eur. J. Immunol. 20, 1137–1142 [DOI] [PubMed] [Google Scholar]
- Naka T., Narazaki M., Hirata M., Matsumoto T., Minamoto S., Aono A., Nishimoto N., Kajita T., Taga T., Yoshizaki K., Akira S., Kishimoto T. (1997). Structure and function of a new STAT-induced STAT inhibitor. Nature 387, 924–929 10.1038/43219 [DOI] [PubMed] [Google Scholar]
- Nielsen M., Svejgaard A., Skov S., Odum N. (1994). Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of stat3 in human T lymphocytes. Eur. J. Immunol. 24, 3082–3086 [DOI] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I., Smith A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060 10.1101/gad.12.13.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein A. F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R. M. (1999). Control of early viral and bacterial distribution and disease by natural antibodies. Science 286, 2156–2159 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- Pennell C. A., Mercolino T. J., Grdina T. A., Arnold L. W., Haughton G., Clarke S. H. (1989). Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur. J. Immunol. 19, 1289–1295 [DOI] [PubMed] [Google Scholar]
- Rolink A., Grawunder U., Winkler T. H., Karasuyama H., Melchers F. (1994). IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int. Immunol. 6, 1257–1264 [DOI] [PubMed] [Google Scholar]
- Rothstein T. L. (2002). Cutting edge commentary: two B-1 or not to be one. J. Immunol. 168, 4257–4261 [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Kolber D. L. (1988a). Anti-Ig antibody inhibits the phorbol ester-induced stimulation of peritoneal B cells. J. Immunol. 141, 4089–4093 [PubMed] [Google Scholar]
- Rothstein T. L., Kolber D. L. (1988b). Peritoneal B cells respond to phorbol esters in the absence of co- mitogen. J. Immunol. 140, 2880–2885 [PubMed] [Google Scholar]
- Savitsky D., Calame K. (2006). B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J. Exp. Med. 203, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R., Willson T. A., Viney E. M., Murray L. J., Rayner J. R., Jenkins B. J., Gonda T. J., Alexander W. S., Metcalf D., Nicola N. A., Hilton D. J. (1997). A family of cytokine-inducible inhibitors of signalling. Nature 387, 917–921 10.1038/43206 [DOI] [PubMed] [Google Scholar]
- Su S. D., Ward M. M., Apicella M. A., Ward R. E. (1991). The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J. Immunol. 146, 327–331 [PubMed] [Google Scholar]
- Tanguay D. A., Colarusso T. P., Doughty C., Pavlovic-Ewers S., Rothstein T. L., Chiles T. C. (2001). Cutting edge: differential signaling requirements for activation of assembled cyclin D3-cdk4 complexes in B-1 and B-2 lymphocyte subsets. J. Immunol. 166, 4273–4277 [DOI] [PubMed] [Google Scholar]
- Tanguay D. A., Colarusso T. P., Pavlovic S., Irigoyen M., Howard R. G., Bartek J., Chiles T. C., Rothstein T. L. (1999). Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J. Exp. Med. 189, 1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin J. L., Pitard V., Dechanet J., Miossec V., Gualde N., Moreau J. F. (1998). Leukemia inhibitory factor: part of a large ingathering family. Int. Rev. Immunol. 16, 397–426 [DOI] [PubMed] [Google Scholar]
- Tumang J. R., Frances R., Yeo S. G., Rothstein T. L. (2005). Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 174, 3173–3177 [DOI] [PubMed] [Google Scholar]
- Tumang J. R., Hastings W. D., Bai C., Rothstein T. L. (2004). Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur. J. Immunol. 34, 2158–2167 [DOI] [PubMed] [Google Scholar]
- Tumang J. R., Owyang A., Andjelic S., Jin Z., Hardy R. R., Liou M. L., Liou H. C. (1998). c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 28, 4299–4312 [DOI] [PubMed] [Google Scholar]
- Wang H., Clarke S. H. (2004). Regulation of B-cell development by antibody specificity. Curr. Opin. Immunol. 16, 246–250 [DOI] [PubMed] [Google Scholar]
- Wang Z., Morris D. L., Rothstein T. L. (1995). Constitutive and inducible levels of egr-1 and c-myc early growth response gene expression in self-renewing B-1 lymphocytes. Cell. Immunol. 162, 309–314 [DOI] [PubMed] [Google Scholar]
- Wong S. C., Chew W. K., Tan J. E., Melendez A. J., Francis F., Lam K. P. (2002). Peritoneal CD5( B-1 cells have signaling properties similar to tolerant B cells. J. Biol. Chem. 277, 30707–30715 [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Berland R. (2001). Cutting edge commentary: origins of B-1 cells. J. Immunol. 166, 2163–2166 [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., Kubo M. (2007). SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- Zhong X., Gao W., Degauque N., Bai C., Lu Y., Kenny J., Oukka M., Strom T. B., Rothstein T. L. (2007a). Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur. J. Immunol. 37, 2400–2404 10.1002/eji.200737296 [DOI] [PubMed] [Google Scholar]
- Zhong X., Tumang J. R., Gao W., Bai C., Rothstein T. L. (2007b). PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur. J. Immunol. 37, 2405–2410 10.1002/eji.200737461 [DOI] [PubMed] [Google Scholar]