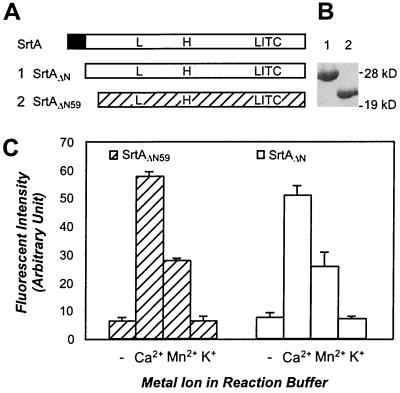
Figure 1.
Truncated sortase enzymes, SrtAΔN and SrtAΔN59, cleave LPXTG peptides and are activated by calcium ions. (A) Primary structure of wild-type sortase (SrtA), SrtAΔN (1) and SrtAΔN59 (2) and the position of the conserved leucine (L97), histidine (H120), and cysteine (C184) residues. The N-terminal membrane anchor of S. aureus SrtA is indicated as a black box. (B) Coomassie blue-stained SDS/PAGE displays the migration of purified SrtAΔN (lane 1) and SrtAΔN59 (lane 2). (C) SrtAΔN59 and SrtAΔN were incubated with d-LPETG-e peptide, and cleavage was measured as an increase in fluorescence. Addition of 2 mM Ca2+ or Mn2+ ions to the reaction buffer stimulated enzyme activity 8-fold, whereas K+ had no effect on cleavage. (− marks control.)