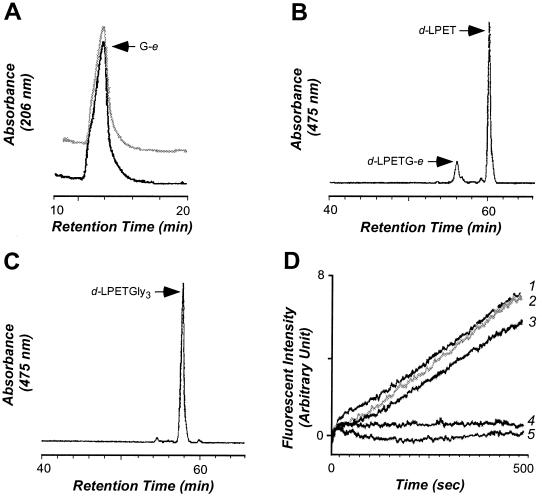
Figure 2.
Truncated sortase SrtAΔN59 catalyzes the transpeptidation reaction of surface protein anchoring. (A) SrtAΔN59 was incubated with the polypeptide substrate d-LPETG-e in the presence or absence of peptidoglycan substrate (NH2-Gly3), and reaction products were separated by RP-HPLC on C18 columns (see Experimental Procedures). Products that eluted at 14 min (1% acetonitrile) were analyzed by electrospray ionization-MS, revealing the sequence G-e. (B) The fluorescent product (475 nm) generated during the absence of NH2-Gly3 eluted at 61 min (76% acetonitrile) and is composed of d-LPET. (C) Fluorescent product generated in the presence of NH2-Gly3 eluted at 58 min (59% acetonitrile) with the structure d-LPET-Gly3. (D) SrtAΔN59 catalyzes the transpeptidation reaction in the presence of 5 mM NH2-Gly3 (trace 1) at a faster rate than hydrolysis of the substrate peptide d-LPETG-e in the absence of the peptidoglycan substrate (trace 3). Transpeptidation is inhibited by 5 mM MTSET, a methyl methanethiosulfonate reagent (5). As a control, we measured the rate of transpeptidation when SrtAΔN (trace 2) or no enzyme (trace 4) was added. Reaction mixtures contained 5 μM d-LPETG-e and 10 μM SrtAΔN or SrtAΔN59 in 520 μl of buffer R at 37°C.