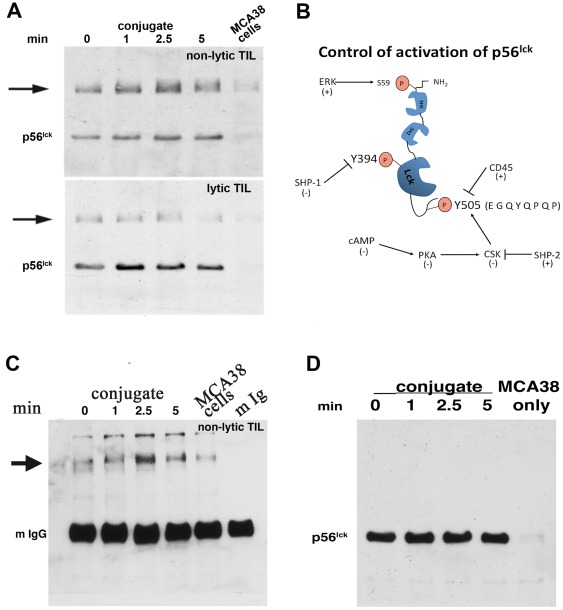
Figure 1. Reciprocal immunoblot analysis of p56lck isolated from nonlytic and lytic MCA38 TIL.
(1a) TIL were purified as described in [6] and either immediately used to form conjugates with MCA38 cells for the indicated times (upper panel) or plated in complete RPMI medium (lower panel) until performance of conjugation and reciprocal immunoblotting. Following incubation, detergent lysates were prepared and immuneprecipitated with Ab reactive with an epitope in the amino terminal portion of the protein (clone 3A5). Immuneprecipitated p56lck was subjected to immunoblotting using anti-p56lck (pY505) and detected by chemiluminescence following reaction with peroxidase-conjugated anti-rabbit. (1b) Schematic diagram of p56lck. The two primary sites of p56lck regulation- the kinase activation motif (centered at Y394) and the inhibitory motif (centered at Y505) are indicated by boxes and key regulators of Y phosphorylation at each site are indicated. ‘+’ and ‘−’ indicate whether a given enzyme causes activation or inhibition of p56lck activity. Activation of kinase function is mediated by phosphorylation of Y394 which is autophosphorylated upon dephosphorylation of Y505 (by CD45). Once phosphorylated, control of kinase function is mediated by Shp-1 dephosphorylation of Y394. Phosphorylation of Y505 (by Csk) prevents autophosphorylation of Y394 and Csk activity is controlled by cAMP regulation of PKA activity. The amino acid sequence of the Y505 motif is also indicated. (1c) Reciprocal immunoblot analysis of p56lck using anti-pY Ab. TIL:MCA38 conjugates were formed for the indicated times as described above, extracts were prepared and immuneprecipitated with anti-p56lck (Ab 3A5), and blotted with anti-pY (4G10). The position of mouse IgG (the species used for p56lck immuneprecipitation, indicated as ‘mIgG’, and which obscures p56lck) is validated by analysis of purified mouse IgG in the last gel lane and the arrowhead indicates the interacting protein detected by anti-pY blotting. (1d) High mw band is not p56lck dimer. Substitution of anti-pY505 with an anti-p56lck reactive to a different p56lck epitope than 3A5 (Ab 2102) was performed and detected only p56lck migrating at the appropriate mw, and not a higher molecular weight species, eliminating the possibility that the higher mw band represented dimeric p56lck.