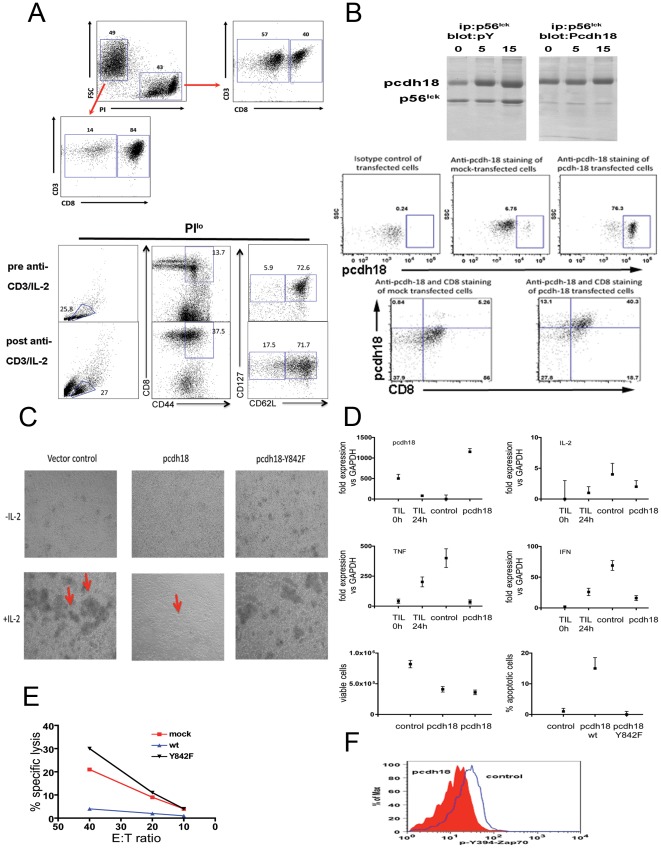
Figure 6. Biochemical and functional analyses of lytic T cells transfected with pcdh18.
(6a) Flow cytometry analysis of primary lytic effector cells generated from spleens of 7 week old mice (prepared as described in ‘Materials and Methods’). PIlo cells are >80% CD8+, PIhi cells are ∼40% CD8+ (top panel). >70% of CD8+CD44hi cells are CD62LhiCD127hi (bottom panel). (6b) (top) Reciprocal immunoblot of p56lck isolated from nonlytic TIL. Analysis was performed as described in Figure 1a (after conjugation with cognate MCA38 tumor cells for 0, 5, or 15 min as indicted and immune precipitated with anti-p56lck Ab 2102- left panels- or Ab 3A5- right panels) and blots were probed with anti-pY or anti-Pcdh18 as indicated. (bottom) Expression of pcdh18 protein in transfected effector cells by flow cytometry was as described in ‘Materials and Methods’. Cells were stained with control or anti-pcdh18 Ab as indicated (top). (6c) Phase contrast microscopy of transfected cells. Effector cells were transfected as indicated and cultured in vitro in the presence or absence of IL-2 for ∼24 h before microscopy. Arrows indicate cell clusters. (6d) RNA was extracted from transfected effector cells (‘control’ or ‘pcdh18’), nonlytic TIL (‘TIL 0 h’), or lytic TIL that were activated with anti-CD3 for 4 hours (‘TIL 24 h’) and used for cytokine qRT-PCR, or cells were assessed for viability (PI and Annexin V staining), as described in ‘Materials and Methods’. In the viability assay two different plasmid constructs were used in separate experiments (bottom left panel) or the Y842F mutant (bottom right panel). (6e) Transfected effector cells were assayed for lytic function by re-directed cytolysis assay as described in ‘Materials and Methods’. (6f) Transfected cells were assayed for binding of anti-Zap70 pY493 after permeabilization following activation with anti-TCR for 2 min as described in ‘Material and Methods’.