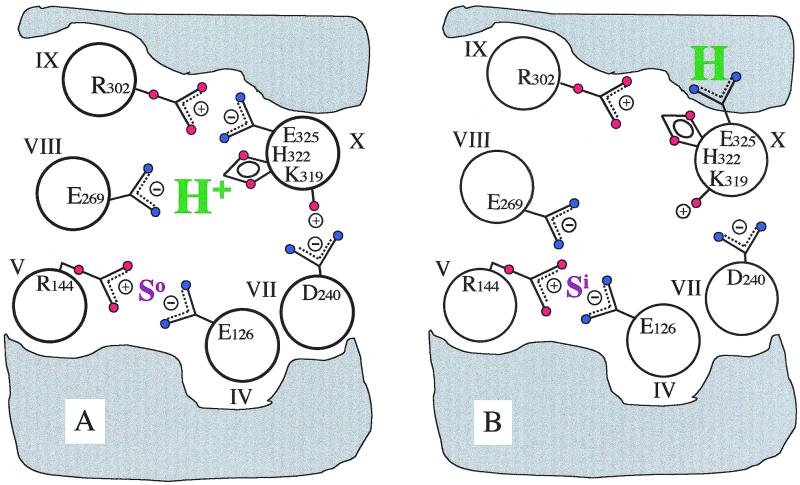
Figure 1.
Model for H+ translocation during lactose/H+ symport via lac permease. For clarity, 6 of the 12 helices that compose the permease are shown. The gray area designates the low dielectric environment of the lipid bilayer. (A) In the ground-state conformation, the relevant H+ is shared by His-322 (helix X) and Glu-269 (helix VIII), whereas Arg-302 (helix IX) is charge-paired with Glu-325 (helix X). In this conformation, lac permease binds substrate with high affinity at the outer surface (So). Glu-126 (helix IV) and Arg-144 (helix V) are charge-paired and represent the major components of the substrate-binding site. Also shown is the charge-pair between Asp-240 (helix VII) and Lys-319 (helix X), which are not essential for the mechanism. (B) Substrate binding induces a conformational change that disrupts the E269/H322 and R302/E325 charge-pairs and leads to the transfer of the H+ to Glu-325, now stabilized by the low dielectric environment. At the same time, the substrate-binding site becomes exposed to the inner surface of the membrane (Si). After substrate dissociation, Glu-325 deprotonates at the inside surface (because of the rejuxtaposition of Glu-325 with Arg-302) as the permease relaxes back to the ground-state conformation.