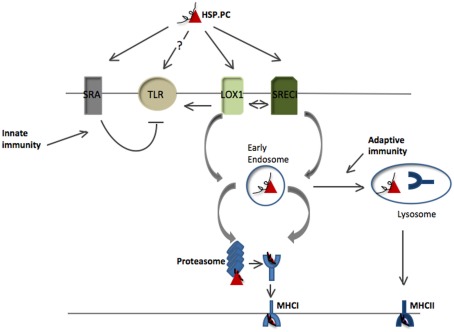
Figure 1.
Antigen presentation pathways for HSP-bound polypeptides. HSP.PCs bind to surface receptors on DC including SRECI and LOX-1 and are internalized in complexes with the receptors. Binding to these receptors may also trigger secondary activation of Toll-like receptors (TLR2 or TLR4) that may amplify antigen cross presentation. Alternatively some studies suggest direct binding of HSPs to TLR. HSP binding to cell surface SRA/CD204 is inhibitory to TLR4 activity and likely antagonizes the immune responses induced by HSPs through LOX-1 or SRECI. HSP.PC are internalized by SRECI or LOX-1 into endosomes with the subsequent release of the peptides from the HSP.PC complex. Such peptides are then processed either within endosomes or undergo trafficking to the cytosol where they encounter the proteasomal system. Processed peptides are then loaded either onto MHC class I in the ER or phagosomes or onto MHC class II molecules in lysosomes and presented to, respectively CD8+ T cells and CD4+ T cells. The triage mechanisms directing HSP-chaperoned peptides toward either of these two pathways are currently not known.