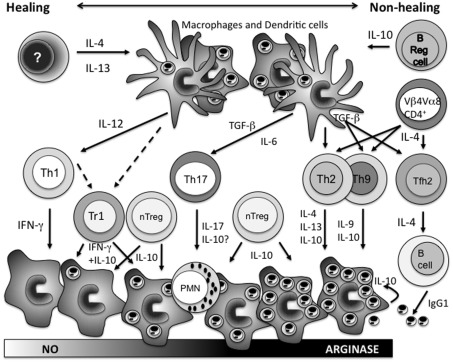
Figure 1.
The mechanisms that influence the expansion of different CD4+ T cell populations as part of the adaptive immune response following Leishmania major infection, and their role in determining the outcome of disease. Early IL-4 (IL-13) instructs DCs to produce IL-12 that together with antigen presentation drives the expansion of Th1 cells. IFN-γ production from Th1 cells induces classical macrophage activation, nitric oxide production, and parasite killing. Latent infection, but also in more virulent models, persistent infection is maintained by type-1 regulatory cells producing both IFN-γ and IL-10, and natural T regulatory cells producing IL-10. In the absence of early IL-4, B regulatory cells producing IL-10 instruct the inhibition of DC IL-12 production allowing the expansion of non-Th1 populations. Under the influence of TGF-β, expansion of Th17 cells causes neutrophil influx into lesions which is often associated with increased pathology. Expansion of Th2, Th9, and Tfh2 cells is further facilitated under the influence of IL-4 from Vβ4Vα8 CD4+ T cells responding to the parasite LACK antigen. Type-2 cytokines from Th2/Th9 populations induce alternative macrophage activation, increased arginase expression, and parasite expansion. Tfh2 cells induce B cell IgG1 production and IgG1 opsonized parasites via macrophage FcγR uptake stimulates host cell IL-10 release which together with the influence of cytokines from the other non-Th1 CD4+ T cell populations promotes progressive disease. Overall depending on the virulence of the L. major parasite strains and the animal model examined these various CD4+ T cell populations interact to create a dynamic disease spectrum. Studies on L. donovani infections would suggest that type-2 responses are not disease promoting for visceral leishmaniasis while those with L. mexicana would suggest a significant role.