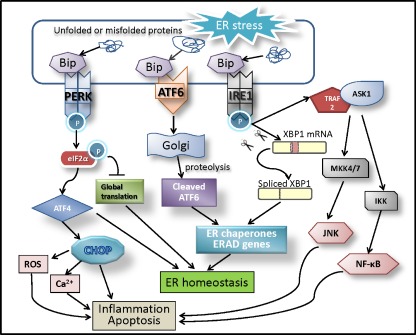
Fig. 1.
ER stress-driven UPR signal pathways in inflammation and apoptosis. Accumulation of unfolded and misfolded proteins in the ER lumen (ER stress) is sensed by three ER membrane proteins (ER stress transducers)—IRE1, PERK, and ATF6. In response to ER stress, GRP78 dissociates from ER stress transducers and binds to unfolded and misfolded proteins, resulting in the activation of ER stress transducers. Upon activation, IRE1 splices the mRNA of XBP1 and produces an active transcription factor named spliced XBP1 (XBP1-S). The activation of PERK increases phosphorylation of eIF2α leading to a global attenuation of protein synthesis and a concomitant increase in ATF4 translation. ATF6 dissociated from GRP78 translocates to the Golgi apparatus where it is cleaved by proteolysis, and becomes an active transcription factor. XBP1 and ATF6 transcriptionally upregulate ER chaperones and proteins implicated in the ER-associated protein degradation (ERAD), which reduce ER stress and restore ER homeostasis. During prolonged ER stress, ATF4 induces CHOP expression, which increases reactive oxygen species (ROS) generation, promotes calcium influx from the ER to mitochondria, and activates inflammatory and proapoptotic cascades. In addition, IRE1 recruits TRAF2 and ASK1, resulting in JNK and NF-κB activation. These events contribute to pathological inflammation and apoptosis