Table 1.
Glyoxylate Stetter Substrate Scopea
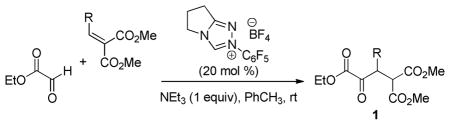 | |||
|---|---|---|---|
| Entry | R | Product | % yieldb |
| 1 | C6H5 | 1a | 96 |
| 2 | 4Cl-C6H5 | 1b | 95 |
| 3 | 4Me-C6H5 | 1c | 92 |
| 4 | 4MeO-C6H5 | 1d | 93 |
| 5 | 4NC-C6H5 | 1e | 90 |
| 6 | 2Me-C6H5 | 1f | 78 |
| 7c | piperonyl | 1g | 91 |
| 8 | 2-furyl | 1h | 87 |
| 9 | 3-N-TsIndole | 1i | 89 |
| 10 | 3-N-BocIndole | 1j | 84 |
| 11 | CH2CHPh | 1k | 93 |
Conditions: Unless otherwise noted, all reactions were performed on a 2.0 mmol scale in PhCH3 (4 mL) at ambient temperature for 16 h.
Isolated yield.
Reaction performed on a 10 mmol scale.
