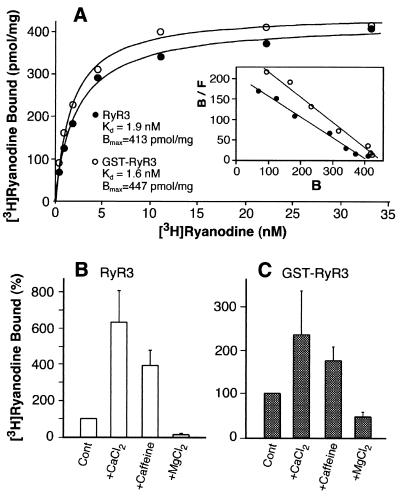
Figure 2.
Purified recombinant RyR3 and GST-RyR3 proteins exhibit regulated, high-affinity [3H]ryanodine binding. (A) Equilibrium [3H]ryanodine binding to purified RyR proteins was carried out as described in Materials and Methods. The purified GST-RyR3, which was from the same preparation that was used for cryo-EM and 3D reconstructions, exhibits a Kd of 1.6 nM and Bmax of 447 pmol/mg protein. Different preparations of purified RyR3 (glutathione-eluted) were used for [3H]ryanodine binding and for 3D reconstructions. The Kd and Bmax values for the RyR3 were 1.9 ± 0.17 nM and 413 ± 6.3 pmol/mg protein (n = 2). [3H]Ryanodine binding to purified RyR3 (B) and GST-RyR3 (C) was carried out in the presence of 150 nM free Ca2+ (control) or with 0.8 mM Ca2+, 2.5 mM caffeine, or 5 mM Mg2+. Data shown are mean ± average error from three separate experiments. All binding determinations were done in duplicate.