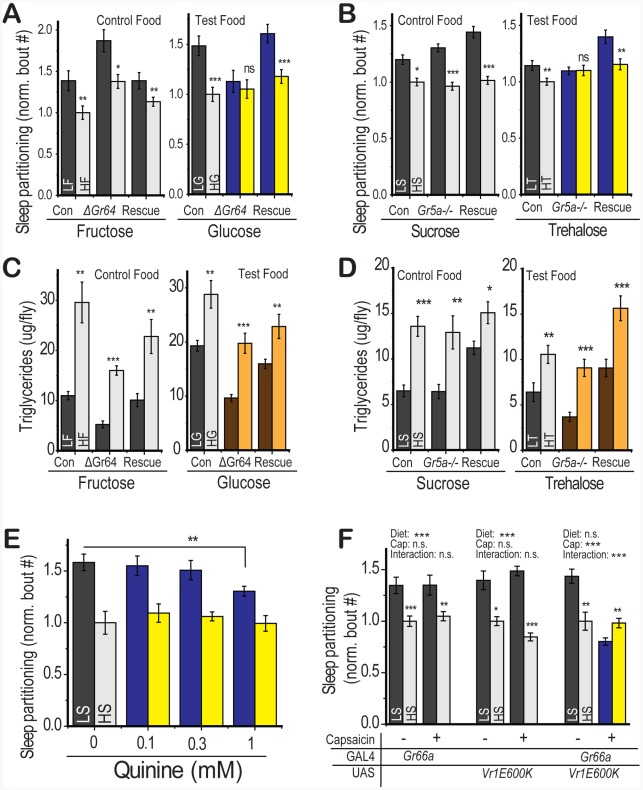
Figure 5. Gustatory inputs mediate sleep partitioning in response to dietary sugar.
For each indicated sugar, flies were tested on a medium with 2.5% or 30% of that sugar on a 2.5% yeast base. (A) Flies with a deletion in the Gr64a-f sweet-sensing cluster retained sensitivity to fructose (control food) but were insensitive to a glucose-containing food (test food). This effect was rescued by expression of a UAS-Gr64abcd_GFP_f construct with a Gr5a-GAL4 promoter in the Gr64 deletion background. (B) Flies with a deletion in the Gr5a trehalose receptor retained sensitivity to sucrose (control food) but were insensitive to a trehalose-containing food (test food). This effect was rescued by expression of a UAS-Gr5a with a Gr5a-GAL4 promoter. Deletion of (C) the Gr64 cluster or (D) Gr5a did not compromise the rapid shift in energy storage following dietary switch on either the control or test food. (E) Quinine supplementation of the food at the indicated concentrations (significance value from one-way ANOVA followed by Fisher LSD within LS group) attenuated the sleep response to LS diet in yw control flies. (F) Genetic stimulation of Gr66a-containing neurons using the Vr1E600K capsaicin receptor in the presence of capsaicin was sufficient to suppress the LS-induced sleep response (significant diet∶capsaicin interaction by two-way ANOVA). There was no effect of capsaicin feeding or diet∶capsaicin interaction in control flies containing either the Gr66a-GAL4 or UAS-Vr1E600K alone but these controls retained a significant response to diet. Error bars represent mean +/− SEM for each group. *** p<0.001, ** p<0.01,* p<0.05 for all statistical calculations. Significance values for t-tests between dietary conditions are shown above each set of bars and significance values for two-way ANOVA are shown above the graph, where applicable. See Figure S4 for additional supporting evidence.