Figure 4. LIC is phosphorylated by BIN2/GSK1.
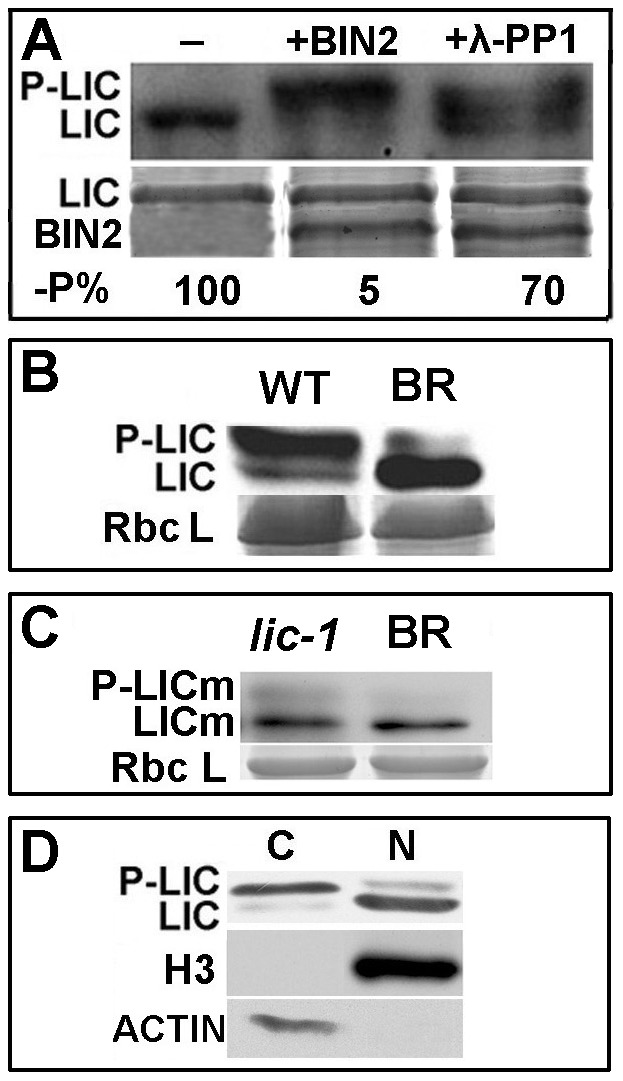
(A) Immunoblotting analysis to demonstrate that LIC was phosphorylated by BIN2. LIC phosphorylation was antagonized by λ-phosphatase 1 (PP1). The phosphorylation status of LIC is illustrated by autoradiography of an anti-LIC antibody in the top panel. The amount of protein is shown with Coomassie Blue staining in the bottom panel. The levels of unphosphorylated LIC relative to the control without BIN2 and PP1 (-P%) were calculated after normalization against the intensity of Coomassie Blue staining, and these values are shown beneath the gel images. (B) Treatment with BR (1 µM) decreased the levels of phosphorylated LIC and increased that of unphosphorylated LIC. Rice plants were grown for 2 weeks and then soaked with 1 µM 24-eBL (+) or mock solution (−) for 3 h. LIC protein was analyzed by immunoblotting with an anti-LIC antibody (upper panel). The loading control with Coomassie Blue staining is shown in the bottom panel. (C) The mutated protein LICm caused decreased phosphorylation in the lic-1 mutant. The 24-eBL concentration was 1 µM. (D) Immunoblotting assay for LIC protein in the nuclear and cytoplasmic fractions. Dephosphorylated LIC was dominant in the nucleus (N), and phosphorylated forms were dominant in the cytoplasm. Nuclear and cytoplasmic protein fractions were extracted from 2-week-old rice seedlings. Histone 3 was a marker for the nuclear protein and ß-actin for the cytoplasmic protein.
