Abstract
Background/Aims
The proper assessment of the current disease status of patients with chronic hepatitis B would be valuable for establishing optimal management strategies.
Methods
The clinical and laboratory characteristics of 2,954 patients with current or previous antiviral treatment (46.2±10.8 years, 69.7% male) enrolled from 46 referral hospitals and 129 local hospitals or clinics throughout Korea were analyzed.
Results
The disease status included chronic hepatitis, cirrhosis, and hepatocellular carcinoma in 79.9%, 16.4%, and 3.7% of the patients, respectively. The major mode of hepatitis B virus (HBV) infection was vertical transmission. The hepatitis C virus (HCV) co-infection rate was 1.5%; however, only 50.8% of patients were evaluated for HCV. The use of herbal or complementary medicines was reported in 33.5% of the patients. The majority of patients (97.6%) were treated with oral nucleoside/nucleotide analogues. Several characteristics were different between the patients treated at referral hospitals and local hospitals/clinics, including the disease state, choice of antiviral drug, and methods of HBV DNA measurement.
Conclusions
This study provides a comprehensive picture of the clinical and laboratory characteristics of patients treated in Korea. Efforts to optimize management strategies are warranted.
Keywords: Hepatitis B virus, Epidemiology, Korea
INTRODUCTION
Korea is an endemic area of hepatitis B virus (HBV) infection.1 The prevalence of HBV infection in Korea was 8% to 15% before the introduction of hepatitis B vaccination in the early 1980s and its integration into universal vaccination for newborn infants in 1995.2,3 The prevalence of hepatitis B surface antigen (HBsAg) among Korean middle school students has markedly decreased from 3.2% in the late 1990s to 0.4% in 2007 and HBsAg positivity in preschool children also decreased from 0.9% in 1995 to 0.2% in 2007.3 However, the chronic HBV infection rate is still high, ranging from 2% to 8.9% in adults.4-6 Until now, HBV infection is a major etiology of liver cirrhosis and hepatocellular carcinoma (HCC) in Korea.1
Since the introduction of lamivudine to Korea in 1999, oral nucleoside or nucleotide analogues has been widely used for patients with chronic hepatitis B (CH-B). Significant changes in characteristics of CH-B patients have been suggested since the introduction of hepatitis B vaccination and antiviral agents in Korea. Proper assessment of characteristics of CH-B patients with antiviral treatment would be valuable for establishing the optimal management strategy for patients with HBV infection. However, most available data were based on non-representative or small sized samples from selected communities. In this study, we aimed to investigate clinical and laboratory features of HBV infected patients with current or past antiviral treatment across Korea to provide a more comprehensive picture of CH-B patients with antiviral therapy.
MATERIALS AND METHODS
1. Patients
From February 2009 to June 2009, a prospective survey was conducted for randomly selected patients aged 18 year or over and had current or past history of antiviral treatment for CH-B in outpatient clinic of 46 referral hospitals and 129 local clinics or hospitals across Korea. Numbers of the study candidates in each hospital were determined based on the geographic distribution of general population and prevalence of CH-B patients with antiviral treatment. Initially, randomly selected 3,218 patients agreed to participate in this study and replied to the questionnaires. Among them, 264 patients were excluded for the following reasons: 1) those who were not followed up for at least 6 months at the respective hospital or clinic (n=32), 2) those who did not received antiviral treatment (n=155), and 3) those who did not complete the survey (n=77). Finally, 2,954 patients were included and analyzed in this study.
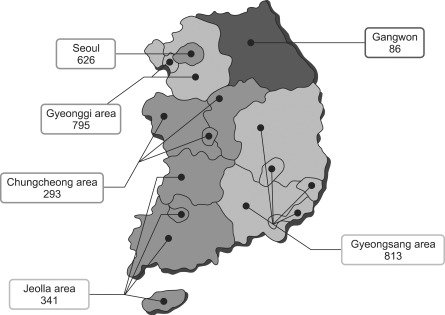
To get the representative samples, we allocated number of patients to be enrolled in each providence according to population statistics of the corresponding area. Patients were enrolled from Seoul (n=626), Incheon and Gyeonggi area (n=795), Gangwon area (n=86), Chungcheong area (Daejeon, Chungcheongnam-do and Chungchengbuk-do; n=293), Gyeongsang area (Pusan, Daegu, Ulsan, Gyeongsangnam-do, and Gyeongsangbuk-do; n=813), and Jeolla area (Gwangju, Jeollanam-do, Jeollabuk-do, and and Jeju island; n=341) (Fig. 1).
Fig. 1.
The geographic distribution of the patients enrolled in the study (n=2,954). The patients with chronic hepatitis B were randomly selected from 46 referral hospitals and 129 local clinics or hospitals throughout Korea.
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the Institutional Review Board of each respective hospital and informed consent was obtained before the survey.
2. Questionnaires
Questionnaires were comprised of two parts. First part, recorded by participating patients, included age, sex, use of herbal medicine and complementary medicine, and family history of HBV infection and HCC. Second part, filled by the participating doctors and investigators, consisted of age at diagnosis of chronic HBV infection, type of hospital, disease state, risk factors of infection, method of HBV DNA measurement, and laboratory values. For the disease state, participating doctors were asked to provide current diagnosis of patients, and they selected most appropriate diagnosis from one of these 3 diagnoses (chronic hepatitis, cirrhosis, and HCC), according to the Korean Association for the Study of the Liver (KASL) guideline.7,8 For the risk factors for HBV infection, family history of HBV-related liver disease, sexual contact, intravenous drug use, and transfusion were assessed. For the laboratory values, alanine aminotransferase (ALT), aspartate aminotransferase (AST), hepatitis B e antigen (HBeAg), and HBV DNA level were assessed at the nearest time point before starting antiviral treatment. HBeAg, ALT, AST, bilirubin, albumin, prothrombin time (PT) and platelet count was obtained at the nearest time of survey. Anti-HCV was also assessed, and analyzed.
3. Data analysis
The possible mode of infection was classified into 1) vertical transmission (from an infected mother to her baby), 2) horizontal transmission (from one family member to another except mother-to-baby transmission), 3) other parenteral risk factors (history of sexual contact, IV drug use, or transfusion), and 4) unknown risk factors. Participating medical institutions were classified into referral hospitals and local clinics/hospitals. Clinical and laboratory characteristics were evaluated according to type of hospitals and disease status.
Statistical analysis was performed using the chi-square to compare discrete variables and the t-test or ANOVA for continuous variables using PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered to be significant.
RESULTS
1. Clinical and laboratory characteristics of the patients
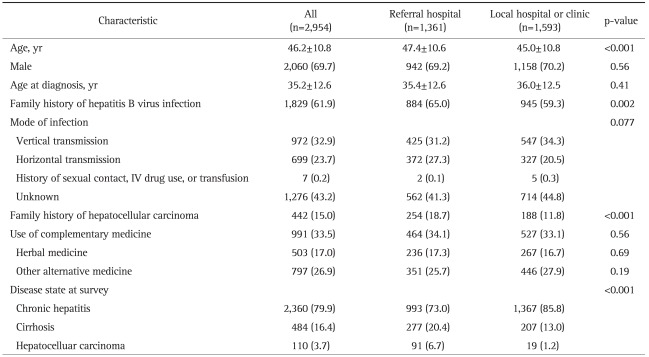
Age of 2,954 patients was 46.2±10.8 years (mean±SD) and male-to-female ratio was 2.3:1 (Table 1). HBV infection was diagnosed at age of 35.2±12.6 years (mean±SD). Family history of HBV infection was noticed in 1,829 (61.9%) patients. Siblings of the patients were reported to be most frequently affected by HBV infection (36.5%), followed by mothers (32.9%), children (10.4%), fathers (8.0%), and spouses (2.4%). Vertical transmission was major mode of infection (32.9%) followed by horizontal transmission (23.7%) and other parenteral exposure (0.2%). However, no identifiable risk factors were noted in 43.2%. Family history of HCC was reported in 442 cases (15.0%). Nine hundred and ninety-one (33.5%) patients reported that they had taken herbal or complementary medicine. Disease status was chronic hepatitis (n=2,360, 79.9%), cirrhosis (n=484, 16.4%), and HCC (n=110, 3.7%).
Table 1.
The Clinical Characteristics of the Patients
Data are presented as mean±SD or number (%).
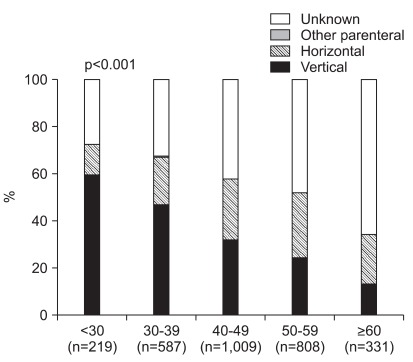
When we further analyzed mode of infection according to age group, vertical transmission was more frequently found at young age. While vertical transmission was reported in 59.4% of patients younger than 30, it was noted in 47.0%, 32.1%, 24.5%, and 13.3% of age groups 30 to 39, 40 to 49, 50 to 59, and 60 or older, respectively (p<0.001). In contrast, the portion of patients with horizontal transmission was 13.2% in age group 18 to 29, it rose with increasing age until reaching its peak of 27.5% in age group 50 to 59 (Fig. 2). Many patients at older ages had unknown mode of transmission.
Fig. 2.
The mode of infection according to age group. The possible mode of infection was different according to age groups. Vertical transmission was the major mode of infection in most age groups, including young patients.
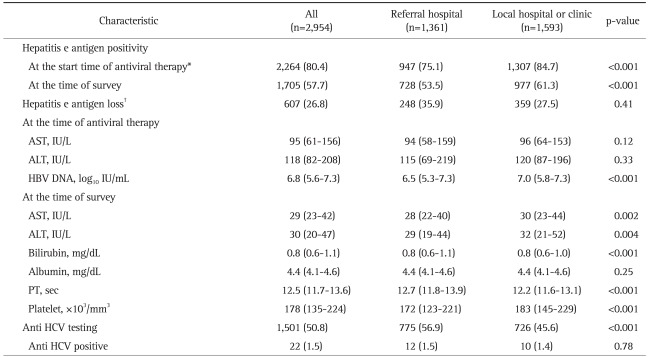
HBeAg was positive in 80.4% of patients at the time of antiviral therapy (Table 2). HBeAg loss was seen in 26.8% of treated patients at the time of survey. Anti-HCV antibody was positive in 22 (1.5%) of 1,501 tested patients, but however, only 50.8% patients had evaluation for HCV.
Table 2.
The Laboratory Characteristics of the Patients
Data are presented as the median (quartile) or number (%).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBV, hepatitis B virus; PT, prothrombin time; HCV, hepatitis C virus.
*A total of 2,817 patients (95.4%) had an available hepatitis B e antigen status at the time of antiviral therapy; †A total of 2,264 patients were hepatitis B e antigen-positive.
2. Treatment history of patients
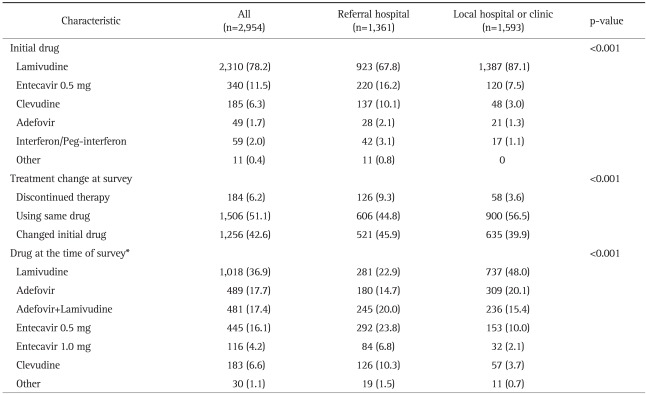
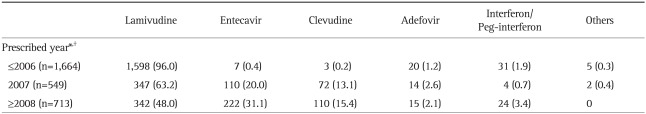
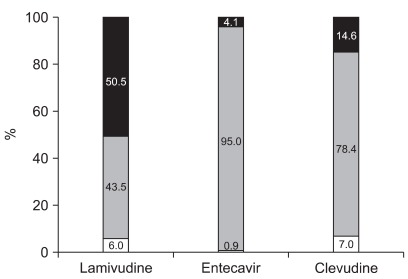
Majority of patients (97.6%) have started therapy with oral nucleoside/nucleotide analogues (Table 3). Lamivudine comprised major proportion, however, initial drug of choice was rapidly changing according to prescribed year (Table 4). Use of entecavir and clevudine was increasing, while use of lamivudine was decreasing (p<0.001). At the time of survey, 51.1% are using the initial drug, 42.6% are using changed drug, and 6.2% discontinued therapy (Table 3). Many patients had changed the drug during therapy. Almost half of patients treated with lamivudine (50.5%) had changed their medication to other medications (Fig. 3). At the time of survey, use of adefovir significantly increased (35.1% as adefovir monotherapy or adefovir and lamivudine combination therapy) when compared to the start time of antiviral therapy (1.7%, p<0.001).
Table 3.
The Antiviral Treatment Characteristics of the Patients
Data are presented as number (%).
*A total of 2,762 patients were being treated at the time of the survey.
Table 4.
The Initial Drug Used for Antiviral Therapy according to the Year Prescribed and Hepatitis B e Antigen Status
Data are presented as number (%).
*Data were not available for 28 patients (0.9%); †p-value <0.001.
Fig. 3.
Changes in the treatment at the time of survey. Almost half of the patients treated with lamivudine had changed medications at the time of survey. Black, gray, and white represent the patients who changed to another drug, used the initial drug prescribed, and discontinued therapy at the time of survey, respectively.
3. Characteristics according to disease status and HBeAg
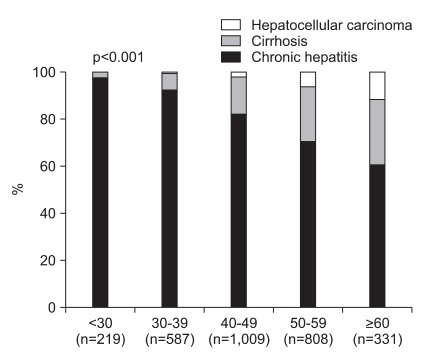
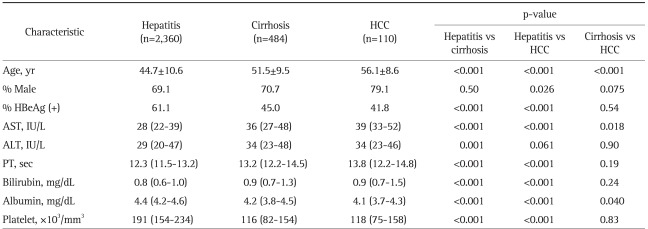
While chronic hepatitis was more common at young age, advanced liver diseases were prevalent at old age. The proportion of patients with cirrhosis and HCC rose as their age increased (2.3%, 7.0%, 15.7%, 23.3%, and 27.8% for cirrhosis, and 0%, 0.5%, 2.0%, 6.1%, and 11.5% for HCC in age group 18 to 29, 30 to 39, 40 to 49, 50 to 59, and ≥60 years, respectively, p<0.001 for all) (Fig. 4). When laboratory results were compared among the patients with different disease status, cirrhosis and HCC were associated with less frequent HBeAg positivity compared to hepatitis (Table 5). Patients with cirrhosis and HCC showed higher AST level, prolonged PT, high bilirubin level, lower albumin level, and low platelet count (Table 5).
Fig. 4.
The disease status according to age group. Although chronic hepatitis was dominant in young patients, cirrhosis and hepatocellular carcinoma were more prevalent in older patients.
Table 5.
The Patient Characteristics at the Time of the Survey according to Disease Status
The values [mean±SD or median (quartile)] were obtained at the nearest time point prior to the survey.
HBeAg, hepatitis B e antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase, PT, prothrombin time.
When compared according to HBeAg status, HBeAg (+) group was younger than HBeAg (-) group (45.1±10.6 vs 50.6±10.1 years, mean±SD, p<0.001). HBV DNA levels of HBeAg (+) group was higher than those of HBeAg (-) group (6.7±1.4 vs 5.8±1.4 log10 IU/mL, mean±SD, p<0.001). The portion of patients with advanced liver diseases was lower in HBeAg (+) group than those of HBeAg (-) group (15.8% vs 36.3%, p<0.001).
4. Characteristics according to hospital type
Overall 1,361 patients (46.1%) were enrolled from referral hospitals and 1,593 patients (53.9%) were enrolled from local clinics/hospitals. Several characteristics differed according to hospital type. Patients from referral hospitals were older, had more patients with family history of HBV infection or HCC, and had more patients with cirrhosis or HCC compared to patients seen at local hospitals/clinics (Table 1). The HBeAg status at the start of therapy, HBeAg status at the time of survey, serum levels of HBV DNA was also significantly different, as well as for several laboratory variables (Table 2). Lamivudine was more frequently used in local hospitals/clinics (Table 3). Regarding methods for measuring HBV DNA level, real-time PCR was used in 41 (89.1%) of 46 referral hospitals, but only in 38 (29.5%) of 129 local clinics/hospitals (p<0.001, Table 6).
Table 6.
The Methods of HBV DNA Measurement Used in Referral Hospitals and Local Clinics/Hospitals
p<0.001.
HBV, hepatitis B virus; PCR, polymerase chain reaction.
DISCUSSION
The optimal management strategy of HBV infection might be different according to nation or respective regions. In this perspective, it is crucial to know current state of CH-B patients with antiviral treatment in the respective region. This study is the first, nation-wide survey that provides the clinical and laboratory characteristics of patients with current or past antiviral treatment for hepatitis B in Korea.
HBV is transmitted parenterally by contaminated blood or other body fluids through blood vessels, skin or mucous membrane.9 The major mode of HBV infection is different by countries. In areas of low endemicity, most HBV infections are acquired through intravenous drug use or unprotected sexual activities, while in areas of high endemicity, perinatal transmission is major mode.10 Perinatal transmission was the most important route in Korea,9 but after universal HBV vaccination program in Korea, shift in primary mode of HBV infection is expected. Nevertheless, this data shows that the major mode of HBV infection in CH-B with antiviral treatment is still vertical transmission (32.9%). HBV vaccination was integrated into universal vaccination program since 1995 in Korea.3 Hence, we can hardly expect a significant preventive effect of vaccine against vertical transmission in the studied patients. Notably, no known risk factor for HBV infection was reported in a significant portion of patients (43.2%). Ignorance or underreporting of risk factors by the patients is suggested. More efforts are warranted to get information on possible mode of infection from these patients, including maternal infection, sexual contact, or intravenous drug use.
HCV co-infection rate among our patients was 1.5%. The HCV co-infection rates in Korean CH-B vary with study population. While co-infection rate of 23% was reported among intravenous drug users,11 0.15% was noted by health promotion center controls.12 Possibility of HCV coinfection should be considered when we monitor the clinical course or consider antiviral treatment in CH-B patients.7,13 However, the test result of anti-HCV was available for only 50.8% of enrolled patients. Our data indicated that we should pay more attention to HCV coinfection in our clinical practice for CH-B patients.
We also noticed that significant proportion of patients (33.5%) had taken or were taking herbal or complementary medicines. Arbitrary use of unauthorized drug can lead to side effect including liver toxicity, drug interaction, or high cost in CH-B with antiviral treatment. It is momentous that physicians should be aware of use of these medicines by their patients.14
Patterns of antiviral therapy also differ by region. For example, adefovir-lamivudine combination therapy is used in 30% of nucleoside-naïve patients in France.15 The use of antiviral agents in this study was collected from all-across Korea, which may give comprehensive picture of current status of antiviral therapy in Korea. Nevertheless, the data about antiviral therapy needs careful interpretation. This study did not intend to evaluate efficacy or outcome of antiviral therapy, and patients started therapy at various time points, for example, patients who started antiviral therapy in earlier times had only one licensed drug (lamivudine). Nevertheless, several points can be drawn from this data. First, most patients in Korea are treated with nucleoside/nucleotide analogues. Second, patients who are using adefovir dramatically increased at the time of survey compared to the start time of antiviral therapy, indicating that lamivudine experience and/or resistant patients may pose a significant proportion of patients in near future. Third, significant proportion of patients was receiving adefovir monotherapy at the time of survey. As most patients had started therapy with lamivudine, majority of them is expected to be lamivudine-resistant patients, who are receiving sequential monotherapy. There are reports of multi-drug resistant mutants in patients who have received sequential monotherapy.16 Hence, multi-drug resistant mutants may emerge as a clinical problem in future. Careful monitoring as well as effort to prevent development of antiviral-resistant HBV may be needed.
We could also notice clinical and laboratory characteristics are different according to disease status and HBeAg status. These are in line with data from previous studies since HBeAg (-) CH-B patients represent a late stage in the course of chronic HBV infection.17,18 We could also notice that clinical and laboratory characteristics are different between patients treated at referral hospitals and patients treated at local hospitals/clinics. Patients treated at local hospitals/clinics were younger, had less advanced disease, and were treated with lamivudine. Furthermore, HBV DNA was measured by real-time PCR in minority of local clinics or hospitals (29.5%). Due to its high sensitivity, specificity, and accuracy and broad dynamic range, use of real-time PCR quantification assays is strongly recommended nowadays.7,13,19 Especially, detection of low level viremia is essential for management of incomplete response or viral breakthrough.7,13,20-22 Adoption of the more sensitive method is strongly warranted even in local clinics or hospitals. The observed differences between referral hospitals and local hospitals/clinics warrant further studies. Most studies in Korea are performed in referral hospitals, and in order to comprehensively understand current status of HBV infection in Korea, more data from local hospitals/clinics is needed.
Our study has a few limitations as a cross-sectional study based on questionnaires, which, in turn, partly depended on retrospective data or memory of the patients and physician. For example, many patients, especially old ones, did not know HBV infection status of their parents. Hence, we might suggest that a significant portion of those with unknown risk factors had been vertically transmitted. Few data sets, such as HBeAg before antiviral therapy, were also incomplete. Since we included only patients with past or current antiviral therapy, population in this study does not represent all the patients with chronic HBV infection. Hence, patient's characteristic would be different if those without antiviral treatement were included. For example, if we included patients in immune-tolerance phase, more patients may have been classified to have been infected by vertical transmission and to be HBeAg positive. Despite a few weak points which were partly inevitable in this kind of study, we could present lots of valuable information on the current epidemiology and laboratory findings in CH-B patients with antiviral therapy.
In conclusion, this study could provide comprehensive understanding about the clinical and laboratory characteristics of patients with antiviral therapy in Korea. Especially, we indicated several problems in current clinical practice for CH-B in Korea, in which imminent improvement is warranted. More efforts to optimize management strategy for patients with HBV infection in Korea are needed based on this data.
Footnotes
This study was supported by GlaxoSmithKline Korea.
References
- 1.Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15(Suppl 6):S13–S24. doi: 10.3350/kjhep.2009.15.S6.S13. [DOI] [PubMed] [Google Scholar]
- 2.Sung JL The Asian Regional Study Group. Hepatitis B virus eradication strategy for Asia. Vaccine. 1990;8(Suppl):S95–S99. doi: 10.1016/0264-410x(90)90227-d. [DOI] [PubMed] [Google Scholar]
- 3.Choe BH. The epidemiology and present status of chronic hepatitis B in Korean children. Korean J Pediatr. 2008;51:696–703. [Google Scholar]
- 4.Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002;17:457–462. doi: 10.3346/jkms.2002.17.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin BM, Yoo HM, Lee AS, Park SK. Seroprevalence of hepatitis B virus among health care workers in Korea. J Korean Med Sci. 2006;21:58–62. doi: 10.3346/jkms.2006.21.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MS, Kim DH, Kim H, et al. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: a cohort study in Korea. Int J Epidemiol. 1998;27:316–319. doi: 10.1093/ije/27.2.316. [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Kim DJ Korean Association for the Study of the Liver Guideline Committee. Management of chronic hepatitis B. Korean J Hepatol. 2007;13:447–488. doi: 10.3350/kjhep.2007.13.4.447. [DOI] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer Study Group; National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 9.Kwon SY, Lee CH. Epidemiology and prevention of hepatitis B virus infection. Korean J Hepatol. 2011;17:87–95. doi: 10.3350/kjhep.2011.17.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 11.Yun H, Kim D, Kim S, et al. High prevalence of HBV and HCV infection among intravenous drug users in Korea. J Med Virol. 2008;80:1570–1575. doi: 10.1002/jmv.21255. [DOI] [PubMed] [Google Scholar]
- 12.Kang J, Cho JH, Suh CW, et al. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90:159–164. doi: 10.1007/s00277-010-1055-5. [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 14.Feldman MS, Friedman LS, Brandt LJ. Sleisenger and Fordtran's gastrointestinal and liver disease: pathology, diagnosis, management. 8th ed. Philadelphia: Saunders; 2006. [Google Scholar]
- 15.Marcellin P, Cadranel JF, Fontanges T, et al. High rate of adefovirlamivudine combination therapy in nucleoside-naïve patients with chronic hepatitis B in France: results of a national survey in 1730 patients. Eur J Gastroenterol Hepatol. 2010;22:1290–1296. doi: 10.1097/meg.0b013e32832fba4f. [DOI] [PubMed] [Google Scholar]
- 16.Yim HJ, Hussain M, Liu Y, et al. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44:703–712. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 17.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2 Suppl 1):S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 18.Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34(4 Pt 1):617–624. doi: 10.1053/jhep.2001.27834. [DOI] [PubMed] [Google Scholar]
- 19.Choi MS, Yoo BC. Management of chronic hepatitis B with nucleoside or nucleotide analogues: a review of current guidelines. Gut Liver. 2010;4:15–24. doi: 10.5009/gnl.2010.4.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology. 2002;122:1554–1568. doi: 10.1053/gast.2002.33428. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]