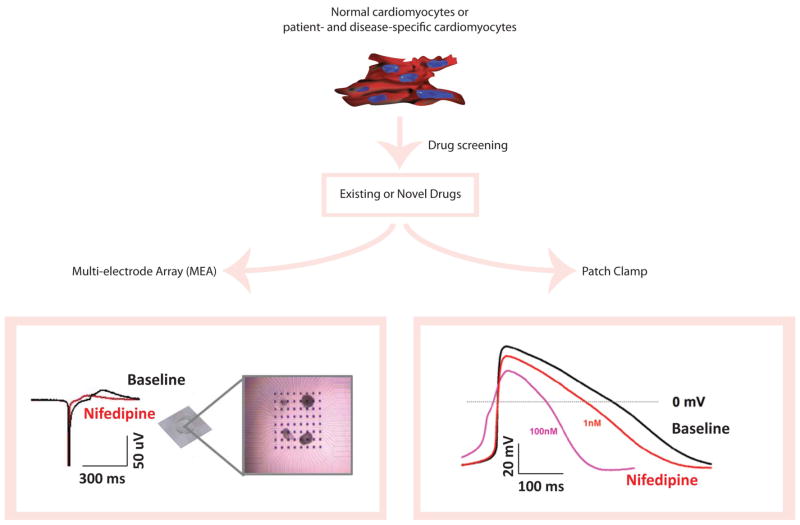
Figure 2. Schematic representation of cardiac drug screening and toxicity testing with human iPSC technology.
Cardiomyocytes are differentiated from normal or disease-specific, patient-derived iPSCs. Subsequently, these iPSC-derived cardiomyocytes (iPSC-CMs) undergo drug screening and toxicity testing with existing or novel drugs. The baseline properties of iPSC-CMs and their response to drugs are determined by electrophysiological assays such as extracellular multi-electrode array (MEA) and patch clamp recordings, using beating embryonic bodies (EBs) in MEA experiments (MED64 MEA amplifier, Alpha Med Scientific, Japan) and isolated single cardiomyocytes in patch clamp recordings (EPC-10 patch clamp amplifier, HEKA, Germany), respectively. Here, nifedipine (100nM) is tested in iPSC-CMs, which showed a significant shortening of field potential duration (FPD) on MEA compared to baseline. Similarly, nifedipine is tested with patch clamp recordings in a dose-dependent manner and exhibited an effect consistent with MEA data.