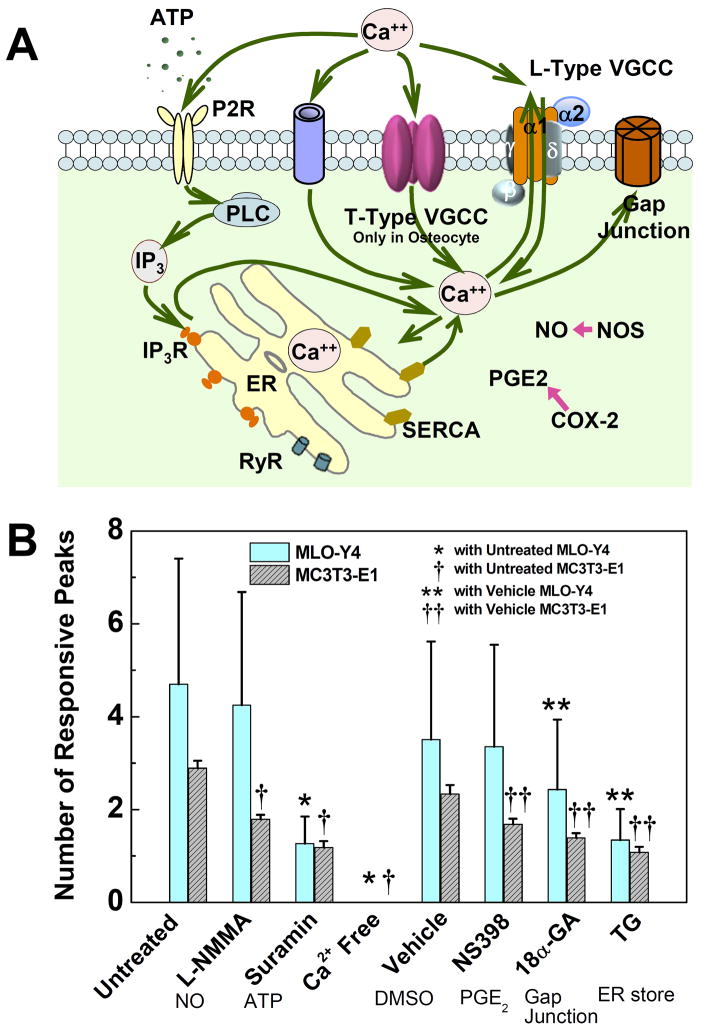
Figure 4.
Roles of critical signaling pathways involved in [Ca2+]i responses were examined and compared between MLO-Y4 and MC3T3-E1 cells. (A) A schematic drawing of calcium signaling pathways in bone cells investigated in present study. The cytoplasm calcium can exchange with extracellular calcium source in medium and intracellular calcium store in ER. Voltage gated calcium channels, ligand gated ion channels (e.g., ATP activated P2X7), G-protein and stretch-gated ion channels can transfer Ca2+ between intra- and extracellular environments. Gap junction provides a direct channel for small molecule (e.g., IP3 and Ca2+) diffusion between connected neighboring cells. The calcium store in ER can be released by activation of IP3 or ryanodine receptors on ER membrane. As two of the most essential signaling pathways involved in bone cell metabolisms, PGE2 and NO pathways were also investigated to identify their correlations with [Ca2+]i responses under mechanical stimulation. (B) The average number of responsive [Ca2+]i peaks from pharmacologically pretreated groups. The affected pathways are listed below the corresponding chemicals. L-NMMA (MLO-Y4: 4.2±2.4; MC3T3: 1.8±0.1), Suramin (MLO-Y4: 1.3±0.6; MC3T3: 1.2±0.1) and Ca2+ free medium (MLO-Y4: 0; MC3T3, 0) treated groups were compared with untreated group (MLO-Y4: 4.7±2.7; MC3T3: 2.9±0.2). NS398 (MLO-Y4: 3.4±2.2; MC3T3: 1.7±0.1), 18α-GA (MLO-Y4: 2.4±1.5; MC3T3: 1.4±0.1) and Thapsigargin (MLO-Y4: 1.3±0.7; MC3T3: 1.1±0.1) treated groups were compared with DMSO vehicle control group (MLO-Y4: 3.5±2.1; MC3T3: 2.3±0.2). The group of Ca2+ free medium had no response to fluid flow. (P < 0.05. Data shown are mean ± std dev.)