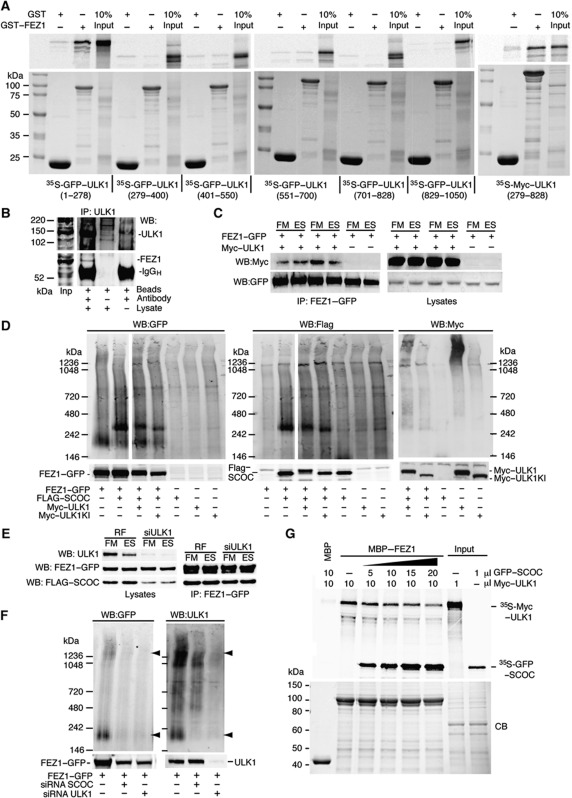
Figure 6.
SCOC regulates FEZ1's interaction with the ULK1 complex. (A) FEZ1 interacts with both the kinase domain (1–278) and the proline–serine rich spacer of ULK1 (279–828). GFP- or Myc-tagged ULK1 fragments were in vitro translated with 35S-methionine and subjected to GST-pulldown assays using GST and GST–FEZ1 purified from E. coli (bottom). Bound proteins were detected by autoradiography following SDS–PAGE (top). (B) ULK1 interacts with FEZ1. ULK1 was immunoprecipitated from HEK293 lysates with anti-ULK1 and bound ULK1 and FEZ1 were detected with anti-ULK1 and anti-FEZ1 antibodies. Input lysate (Inp). (C) The FEZ1–GFP and Myc–ULK1 interaction is unaffected by amino-acid starvation. Anti-Myc and -GFP blots after HEK293 cells were co-transfected with FEZ1–GFP and Myc–ULK1, incubated in FM or ES for 2 h, harvested and the complex was immunoprecipitated using anti-GFP antibody. (D) BN–PAGE reveals SCOC and FEZ1 are in a complex. FEZ1–GFP, FLAG–SCOC and Myc–ULK1 wild type or kinase-inactive ULK1K46I (ULK1KI) were co-expressed in HEK293 cells. Anti-GFP, -FLAG and -Myc western blots are shown for the BN–PAGE gel (top) or the SDS–PAGE gel (bottom). In GFP and FLAG western blots, two different exposures of the same BN–PAGE blot are shown spliced together. (E) siRNA depletion of ULK1 does not affect the interaction of FEZ1 with SCOC. RF or ULK1 siRNA-treated cells were co-transfected with FEZ1–GFP and FLAG–SCOC, then incubated and immunoprecipitated as in (C). Western blot of lysates for ULK1, GFP and FLAG, and immunoprecipitates with GFP and FLAG antibodies. (F) siRNA depletion of SCOC reduces FEZ1–ULK1 complex. HEK293 cells treated with either SCOC or ULK1 siRNAs were transfected with FEZ1–GFP. FEZ1–GFP was detected with anti-GFP in both BN–PAGE and SDS–PAGE. Endogenous ULK1 was detected with anti-ULK1 antibody. Arrowheads indicate a FEZ1–GFP and endogenous ULK1 complex at 200 kDa and a larger MW complex >1236 kDa also detected in (D) in the triple transfection. The 200 KDa complex is sensitive to loss of SCOC and ULK1. (G) SCOC can reduce the interaction of ULK1 with MBP–FEZ1. Immobilised MBP–FEZ1 was incubated with in vitro 35S-methionine-labelled Myc–ULK1. Addition of increasing amounts of in vitro translated 35S-methionine SCOC reduced the amount of Myc–ULK1 bound. Top and bottom panels are as described in (A).