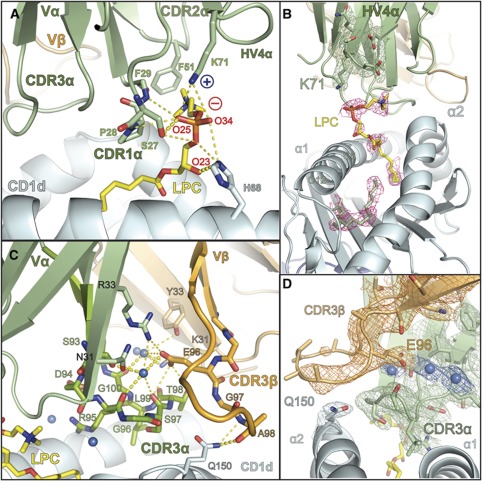
Figure 6.
Specificity of the iNKT TCR for CD1d–LPC is mediated by contacts with the CDR loops of the Vα domain and intricate coordination of the CDR3β loop. (A) The contacts of the iNKT TCR with LPC are mediated exclusively through the CDR1α, CDR2α and HV4α loops shown in green. Side- and main-chain atoms are shown where they establish contacts with the LPC phosphorylcholine headgroup. The positive and negative charge of Lys71 and the phosphate moiety are shown as blue ‘+’ and red ‘−’, respectively. (B) Electron density of the LPC ligand in the CD1d–LPC–iNKT TCR complex. Electron density corresponds to a composite omit map (2Fo−Fc) contoured at 1α around the LPC ligand (pink) and C12 and C6 fatty acids shown in green. Electron density for the HV4α loop and the K71 side chain are shown in green, contoured as with LPC. (C) Hydrogen bonding network of the water-mediated coordination of the CDR3β loop. The CDR3β loop and Vβ domain are shown in light orange, the CDR3α loop and the Vα domain are shown in light green. Stick representation is used to show the side- and main-chain atom contacts between these loops and their coordination by three water molecules, shown as marine coloured spheres. CDR3β's contact with CD1d (coloured cyan), mediated by the Gln150 side chain, is shown as stick representation. LPC is coloured in yellow. In both panels, hydrogen bonds are shown as dashed yellow lines: highly likely H-bonds (>3.3 Å) are shown with heavy dashed lines, probable H-bonds (4 Å> but >3.3 Å) are shown as light dashed lines. (D) Electron density of the CDR3β–CDR3α–CD1d interface. A 2Fo-Fc map is shown, contoured at 1σ around the CDR3β (orange) and the Glu96β side chain, CDR3α (green) and the Gln150 residue of the CD1d α2 helix (cyan). Electron density for three water molecules located in the CDR3α-CDR3β interface is also shown, the waters are represented by spheres coloured in marine.