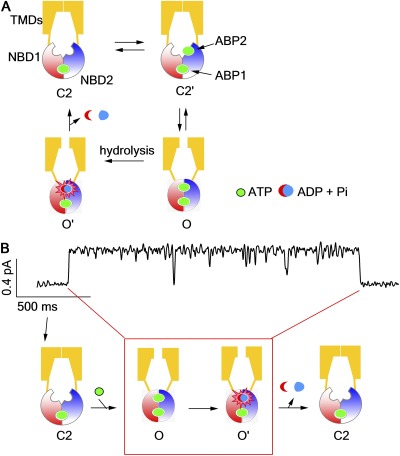
Figure 1.
A hypothetic model of CFTR gated by ATP. (A) A scheme illustrating ATP-dependent gating mechanism of CFTR. This scheme is synthesized based on several of the latest publications on CFTR gating (Csanády et al., 2010; Tsai et al., 2010b; Szollosi et al., 2011). For channel opening, two NBDs dimerize upon ATP binding to the catalysis-competent site (ABP2) in the C2 state, which harbors a partially dimerized NBD with an ATP molecule bound to the catalysis-incompetent site (ABP1). For channel closure, first the ATP in ABP2 is hydrolyzed to convert the prehydrolytic open state (O) to a post-hydrolytic open state (O′). Then, it is the separation of the NBD dimer and the dissociation of hydrolytic products that coincide with gate closure. In the continuous presence of millimolar ATP, CFTR rarely shuttles back to the C1 state, which bears completely separated NBDs. (B) A cartoon depicting how the proposed CFTR gating scheme in A correlates an experimentally observed opening and closing of a single CFTR channel to the molecular events in its NBDs and TMDs. The red box encompasses the open-channel conformations.