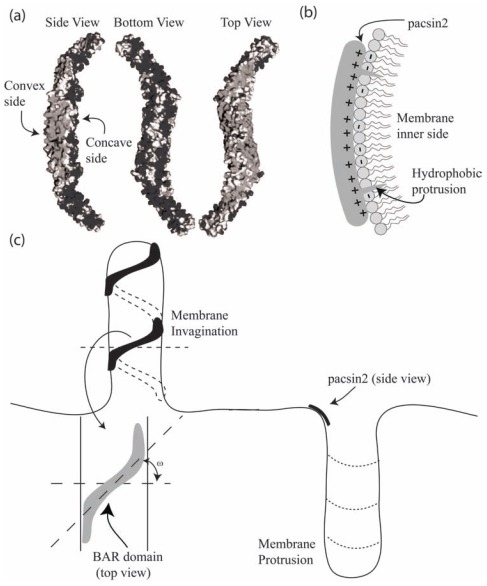
Fig. (1).
The molecular structure and a schematic diagram of pacsin2 EFC/F-BAR domain binding to a cell membrane. (a) Side view of the electric charge surface of pacsin2 dimer. Note that the molecule is positively charged (dark grey) at the concave, whereas the convex side is more negatively charged (light grey) (adapted from [18]). (b) Schematic diagram of pacsin2 EFC/F-BAR domain bound to the membrane. Note that the inner side of the membrane is negatively charged, while the domain contact interface is positively charged. In addition, two hydrophobic protrusions that belong to the BAR domain are docked into the hydrophobic part of the inner membrane leaflet. The binding of BAR domains of positive intrinsic curvature (e.g. pacsin2) to both membrane invaginations and protrusions. ω is the rotation angle of the molecule on the membrane surface. In high concentrations of BAR domains, a spiral aggregate can be formed stabilizing the formation of a membrane invagination.