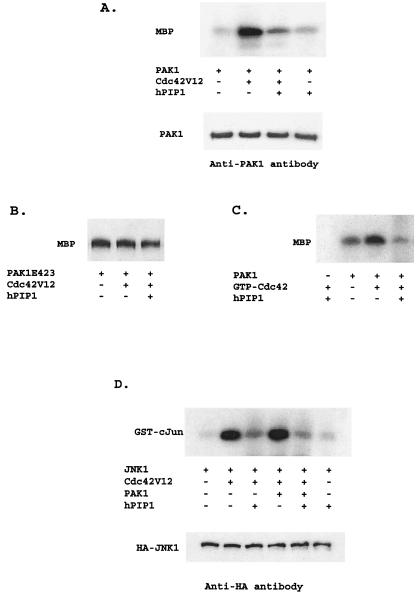
Figure 4.
hPIP1 inhibits Cdc42-stimulated PAK1 activity. (A) Inhibition of Cdc42-stimulated PAK1 activity by hPIP1 in mammalian cells. Cell lysates from COS-7 cells expressing proteins as shown were immunoprecipitated with specific anti-PAK1 antibody to isolate PAK1, and were then subjected to protein kinase assays using myelin basic protein (MBP) as substrate. Bottom band shows that the amount of PAK1 used in the reaction was constant, as assayed by Western blot analysis. (B) hPIP1 had little effect on the kinase activity of PAK1 mutant (PAK1T423E). (C) hPIP1 inhibited GTPγS-Cdc42-mediated PAK1 activity in vitro. A fixed amount of PAK1 was activated by 2 μg of GTPγS-loaded Cdc42 in the presence or absence of hPIP1 (4 μg per reaction) with MBP as a substrate (4 μg per reaction). (D) Inhibition of Cdc42- and PAK1-mediated JNK activation by hPIP1. Cotransfection of hPIP1 greatly inhibited the activation of HA-JNK by Cdc42 (V12) or Cdc42 together with PAK1. JNK kinase was immunoprecipitated by anti-HA monoclonal antibody, and GST-cJun (2 μg, amino acids 1–79) was used as a phosphorylation substrate. Equal amounts of JNK were used in the assays, as shown in the bottom band by Western blot analysis.