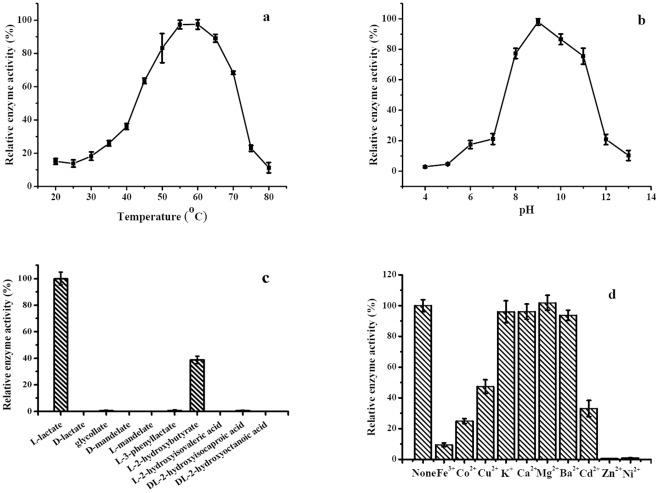
Figure 4. Basic enzymatic properties of l-iLDH from P. stutzeri SDM.
The reaction mixture contained 20 mM l-lactate, 0.2 mM MTT, 50 mM Tris-HCl (pH 7.5), and 0.1 µg purified l-iLDH. (a) Effect of temperature on l-iLDH activity. Enzyme reactions were carried out at pH 7.5 at different temperatures (20–80°C). (b) Effect of pH on l-iLDH activity. The optimum pH was assessed in a buffer containing citric acid, KH2PO4, boric acid, and barbital (CKBB buffer) at various pHs (3.0–13.0) at 30°C. (c) Substrate specificity of l-iLDH from P. stutzeri SDM. The sodium salts of different α-hydroxy acids were used at a concentration of 20 mM. (d) Effect of different metal ions on l-iLDH activity. The concentration of metal ions was 5 mM in 50 mM Tris-HCl (pH 7.5). Values are the average ± SD of three separate determinations.