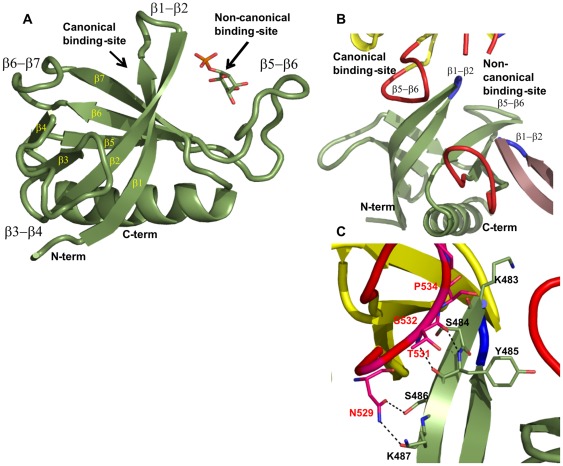
Figure 1. Overall structure of the Slm1-PH domain.
(A) Ribbon representation of the 1.76 Å structure (green) showing that Slm1-PH folds into a seven-stranded β-sheet terminated with an α-helix. All secondary structure elements are labeled in yellow and black. Both canonical and non-canonical binding sites are also marked. (B) In the X-ray crystal structure the canonical binding site is partially occluded by the β5-β6 loop (in red) of the neighboring molecules (in yellow and violet) on both sides. The β1-β2 loops of all the molecules are shown in blue. All the loops in Figure 1B have been smoothened using PyMOL for clarity. (C) Detailed view of the interactions between the β1-β2 loop and the β5-β6 loop of neighboring molecules. Primarily, the side-chain residues of the β5-β6 loop are making contact with the main-chain atoms of the β1-β2 loop region.